
🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
At Infinity Market Research, we dont just deliver data — we deliver clarity, confidence, and competitive edge.
In a world driven by insights, we help businesses unlock the infinite potential of informed decisions.
Here why global brands, startups, and decision-makers choose us:
Industry-Centric Expertise
With deep domain knowledge across sectors — from healthcare and technology to manufacturing and consumer goods — our team delivers insights that matter.
Custom Research, Not Cookie-Cutter Reports
Every business is unique, and so are its challenges. Thats why we tailor our research to your specific goals, offering solutions that are actionable, relevant, and reliable.
Data You Can Trust
Our research methodology is rigorous, transparent, and validated at every step. We believe in delivering not just numbers, but numbers that drive real impact.
Client-Centric Approach
Your success is our priority. From first contact to final delivery, our team is responsive, collaborative, and committed to your goals — because you re more than a client; you re a partner.
Recent Reports
Obesity Management Market
GLP-1 Receptor Agonist Market
Chemotherapy-Induced Neutropenia Treatment Market
Chemotherapy-Induced Neutropenia Treatment Market (By Treatment Type (Antibiotic Therapy, Granulocyte Colony-Stimulating Factor Therapy (G-CSF), Granulocyte Transfusion, Other Treatment Type), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Other Distribution Channel), By Region and Companies)
Aug 2024
Healthcare
Pages: 138
ID: IMR1210
Chemotherapy-Induced Neutropenia Treatment Market Overview
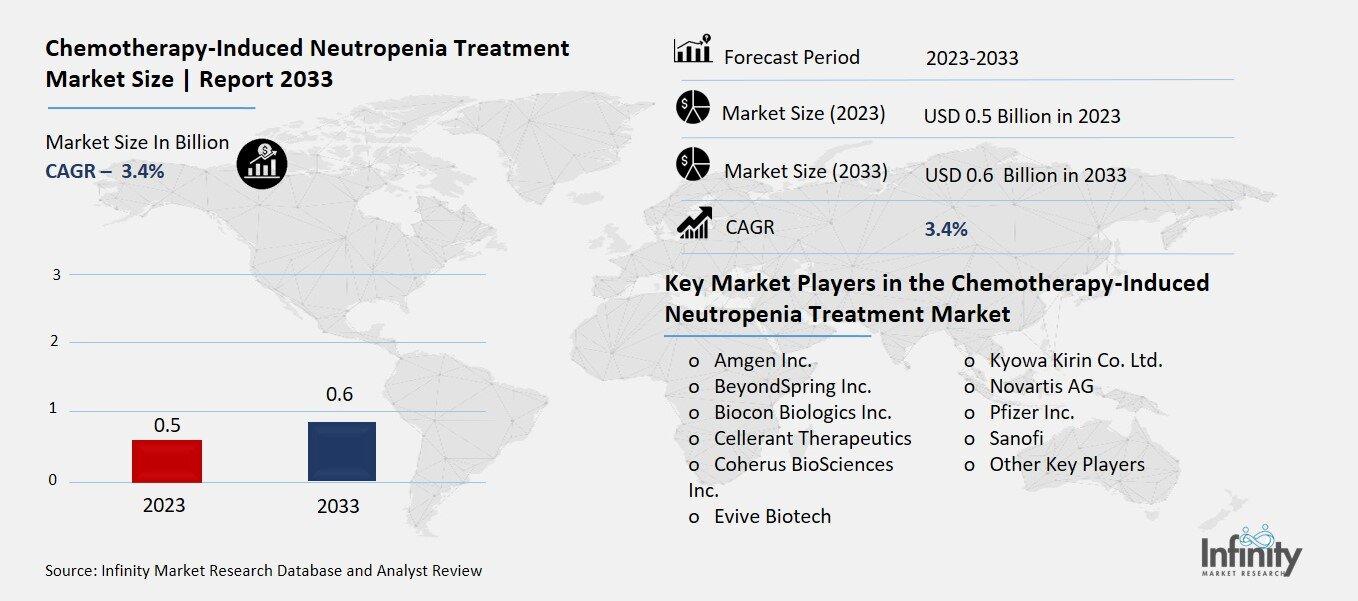
Global Chemotherapy-Induced Neutropenia Treatment Market size is expected to be worth around USD 0.6 Billion by 2033 from USD 0.5 Billion in 2023, growing at a CAGR of 3.4% during the forecast period from 2023 to 2033.
The Chemotherapy-Induced Neutropenia Treatment Market focuses on therapies and treatments for neutropenia, a condition where white blood cell levels drop due to chemotherapy. White blood cells are crucial for fighting infections, and when their numbers are low, patients become highly susceptible to infections. This market includes drugs, therapies, and supportive care that help manage or prevent this condition, ensuring that patients can continue their cancer treatments without severe disruptions.
Companies and healthcare providers involved in this market work on developing solutions that reduce infection risks and improve the overall quality of life for chemotherapy patients. The market includes growth factors like colony-stimulating factors (CSFs), antibiotics, and new therapies aimed at better-managing neutropenia. As cancer treatments evolve and become more aggressive, the demand for effective neutropenia management continues to grow, driving innovation and advancements in this area.
Drivers for the Chemotherapy-Induced Neutropenia Treatment Market
Growing Cancer Incidence
The rising number of cancer cases worldwide is a major driver for the chemotherapy-induced neutropenia (CIN) treatment market. As cancer rates increase, more patients undergo chemotherapy, which often leads to neutropenia. Neutropenia is a condition characterized by a low level of neutrophils; a type of white blood cell essential for fighting infections. This condition requires timely treatment to prevent severe complications. The growing cancer burden is therefore a significant factor driving the demand for effective neutropenia treatments.
Advances in Treatment Options
Recent advancements in medical science have led to the development of new and improved treatments for CIN. Innovative drugs, such as colony-stimulating factors (CSFs), have been designed to boost neutrophil counts and reduce the risk of infections. These advancements offer better efficacy and fewer side effects compared to older treatments. The continuous research and development in this field are expanding treatment options, thus contributing to the market's growth.
Increased Awareness and Early Diagnosis
There is a growing awareness of CIN among healthcare providers and patients. Increased awareness leads to earlier diagnosis and treatment, which is crucial for managing the condition effectively. Early intervention can significantly reduce the risk of severe infections and improve patient outcomes. As awareness campaigns and educational programs become more widespread, the demand for CIN treatments is expected to rise.
Supportive Government Policies and Initiatives
Governments and regulatory bodies around the world are increasingly recognizing the importance of addressing CIN. They are implementing supportive policies and initiatives to improve patient access to treatment. These may include funding for research, subsidies for treatment, and guidelines for managing CIN. Such measures enhance the accessibility and affordability of CIN treatments, driving market growth.
Rising Healthcare Expenditure
Global healthcare expenditure is on the rise, with increased investments in medical research and patient care. Higher healthcare budgets enable hospitals and clinics to invest in advanced treatment technologies and drugs for managing CIN. As healthcare spending grows, so does the ability of healthcare providers to offer effective treatments for neutropenia, thereby boosting market demand.
Development of Personalized Medicine
The shift towards personalized medicine is also influencing the CIN treatment market. Personalized medicine involves tailoring treatment plans based on an individual’s genetic profile and specific needs. This approach can enhance the effectiveness of CIN treatments by targeting the unique aspects of a patient's condition. The growing focus on personalized medicine is driving innovation and expanding treatment options in the CIN market.
Restraints for the Chemotherapy-Induced Neutropenia Treatment Market
Limited Efficacy of Current Treatments
Despite advancements, some existing treatments for CIN may not be effective for all patients. Variability in individual responses to treatment can limit the overall success rate. For example, some patients may not experience a significant increase in neutrophil counts or may suffer from side effects that outweigh the benefits. This variability can hinder the widespread adoption of certain therapies and impact overall market growth.
Regulatory and Approval Challenges
The process of getting new CIN treatments approved by regulatory authorities can be lengthy and complex. Regulatory agencies require extensive clinical trials and data to ensure the safety and efficacy of new drugs. This can delay the introduction of innovative treatments into the market. Additionally, the strict regulatory environment can increase the cost of drug development, which may be passed on to patients.
Market Competition and Price Pressure
The CIN treatment market is competitive, with multiple companies developing and marketing similar therapies. This intense competition can lead to price pressure, as companies may lower their prices to gain market share. While this can benefit patients by making treatments more affordable, it can also affect the profitability of drug manufacturers and impact investment in new drug development.
High Treatment Costs
One of the significant restraints in the chemotherapy-induced neutropenia (CIN) treatment market is the high cost of advanced treatments. Drugs like colony-stimulating factors (CSFs) and other supportive therapies can be expensive, which can limit their accessibility for patients, especially in low-income regions. The high costs can also strain healthcare budgets and insurance coverage, potentially leading to disparities in treatment availability.
Limited Awareness in Low-Income Regions
In many low-income regions, there is limited awareness about CIN and its management. This lack of awareness can result in underdiagnosis and undertreatment of the condition. As a result, patients in these regions may not receive the necessary care or medications, limiting the overall market potential. Addressing this issue requires targeted education and healthcare initiatives to improve awareness and access.
Adverse Effects and Safety Concerns
Some CIN treatments can have adverse effects or safety concerns, which may deter their use. For example, certain drugs may lead to complications such as allergic reactions or bone pain. These side effects can limit the use of these treatments and affect patient adherence. Safety concerns can also impact the approval and acceptance of new therapies, restricting market growth.
Opportunity in the Chemotherapy-Induced Neutropenia Treatment Market
Growing Demand for Effective Treatments
The increasing incidence of cancer worldwide presents a significant opportunity for the chemotherapy-induced neutropenia (CIN) treatment market. As more patients undergo chemotherapy, the demand for effective treatments to manage neutropenia grows. This rising need for effective solutions creates a substantial market opportunity for pharmaceutical companies and biotech firms to develop and market new therapies. The growing patient population ensures a continuous demand for advanced treatment options, driving market expansion.
Innovations in Drug Development
Ongoing research and technological advancements offer promising opportunities in the CIN treatment market. New drug development strategies, including novel biologics and targeted therapies, are being explored to improve treatment efficacy and minimize side effects. Innovations such as long-acting formulations and combination therapies could enhance patient outcomes and provide more options for managing CIN. Companies that invest in cutting-edge research and development are well-positioned to capitalize on these emerging opportunities.
Expansion in Emerging Markets
Emerging markets present a significant growth opportunity for CIN treatments. As healthcare infrastructure improves and economic conditions advance in countries like China, India, and Brazil, there is a rising demand for advanced medical treatments. These regions are experiencing increased cancer incidence and better access to healthcare, creating a growing market for CIN therapies. Expanding into these emerging markets can provide substantial revenue growth and broaden the market reach for CIN treatments.
Increasing Focus on Personalized Medicine
The trend toward personalized medicine offers a unique opportunity in the CIN treatment market. Personalized medicine involves tailoring treatments to individual patient profiles, which can enhance the effectiveness of CIN therapies. By leveraging genetic and molecular information, healthcare providers can offer more precise and effective treatments. This approach not only improves patient outcomes but also creates opportunities for pharmaceutical companies to develop targeted therapies that meet specific patient needs.
Collaborative Research and Partnerships
Collaborations between pharmaceutical companies, research institutions, and healthcare providers can drive innovation and market growth. Strategic partnerships and alliances enable the sharing of resources, expertise, and data, accelerating the development of new CIN treatments. Collaborative efforts can also facilitate clinical trials and bring new therapies to market more efficiently. These partnerships can provide valuable opportunities for companies to enhance their research capabilities and expand their market presence.
Government Support and Funding
Government support and funding for cancer research and treatment development present significant opportunities for the CIN treatment market. Many governments and health organizations are investing in cancer research and providing grants for the development of new therapies. This financial support can help accelerate the development of innovative CIN treatments and improve their accessibility. Companies that align with government initiatives and take advantage of available funding are well-positioned to capitalize on these opportunities.
Trends for the Chemotherapy-Induced Neutropenia Treatment Market
Increasing Use of Biosimilars
One of the notable trends in the chemotherapy-induced neutropenia (CIN) treatment market is the growing adoption of biosimilars. Biosimilars are highly similar to existing biological drugs but are typically more affordable. As patents for original biologic treatments like colony-stimulating factors (CSFs) expire, biosimilars offer a cost-effective alternative while maintaining similar efficacy and safety profiles. This trend helps reduce treatment costs and expands access to CIN therapies, making them more available to a broader patient population.
Focus on Novel Therapies
The market is witnessing a strong emphasis on developing novel therapies for CIN. Researchers are exploring new drug classes, such as novel granulocyte colony-stimulating factors and new combination therapies, to enhance treatment effectiveness and minimize side effects. These innovative approaches aim to address the limitations of current treatments and offer better management options for patients experiencing severe neutropenia. The continuous push for novel therapies reflects the industry's commitment to improving patient outcomes.
Personalized Medicine Approach
The trend toward personalized medicine is becoming increasingly prominent in the CIN treatment market. Personalized medicine involves tailoring treatments based on individual patient characteristics, such as genetic and molecular profiles. This approach allows for more precise targeting of therapies, improving their effectiveness and reducing adverse effects. Personalized medicine is expected to play a significant role in the future of CIN treatment, providing more customized and effective care options for patients.
Rising Adoption of Prophylactic Treatments
Another trend is the rising adoption of prophylactic treatments to prevent CIN rather than just treating it once it occurs. Prophylactic use of drugs like CSFs is becoming more common, especially in high-risk patients undergoing chemotherapy. This proactive approach helps reduce the incidence of neutropenia and associated complications, leading to better patient management and outcomes. The shift towards prophylactic treatments highlights a broader trend in healthcare towards prevention and early intervention.
Integration of Digital Health Technologies
The integration of digital health technologies is transforming the CIN treatment landscape. Tools such as mobile health apps and telemedicine platforms are being used to monitor patients' health remotely, track symptoms, and manage treatments more effectively. These technologies facilitate real-time communication between patients and healthcare providers, improving patient adherence and treatment outcomes. The adoption of digital health solutions reflects a trend towards more connected and patient-centered care.
Growing Emphasis on Patient-Centric Approaches
There is a growing emphasis on patient-centric approaches in the CIN treatment market. This trend involves focusing on the overall patient experience, including addressing quality-of-life issues and managing side effects more effectively. Patient-centric approaches involve incorporating patient feedback into treatment plans, providing education, and supporting patients throughout their treatment journey. This shift towards a more holistic approach aims to enhance patient satisfaction and improve treatment adherence.
Segments Covered in the Report
By Treatment Type
o Antibiotic Therapy
o Granulocyte Colony-Stimulating Factor Therapy (G-CSF)
o Granulocyte Transfusion
o Other Treatment Type
By Distribution Channel
o Hospital Pharmacies
o Retail Pharmacies
o Online Pharmacies
o Other Distribution Channel
Segment Analysis
By Treatment Type Analysis
The Chemotherapy-Induced Neutropenia Treatment Market is divided into four types: antibiotic therapy, granulocyte colony-stimulating factor therapy (G-CSF), granulocyte transfusion, and others. In 2023, the Antibiotic Therapy category dominated the market, accounting for 34.8% of total revenue, owing to its broad use in preventing and controlling neutropenia infections. Antibiotics are frequently administered as preventative measures to limit the incidence of bacterial infections in cancer patients undergoing chemotherapy. Antibiotics also serve an important role in treating existing infections in neutropenic patients. Antibiotics dominate the CIN therapy market due to their great efficacy, low cost, and widespread availability.
Granulocyte colony-stimulating factor (G-CSF) therapy has the highest Compound Annual Growth Rate (CAGR) in the Chemotherapy-Induced Neutropenia (CIN) Treatment Market, owing to its effectiveness in stimulating the production of white blood cells, particularly neutrophils, to prevent and manage neutropenia. With an increasing number of cancer patients receiving chemotherapy and a growing knowledge of the necessity of neutropenia treatment, there is a greater need for G-CSF therapy. Furthermore, innovations in G-CSF formulations and delivery modalities have accelerated its popularity and market expansion.
By Distribution Channel Analysis
Chemotherapy-induced neutropenia Treatment Market segmentation by distribution channel includes Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. In 2023, the Hospital Pharmacies category had the highest revenues. These include hospitals' centralized procurement of drugs and supportive care supplies for cancer treatment, which frequently entails managing chemotherapy-induced neutropenia. Furthermore, hospitals serve as important treatment sites for cancer patients, providing chemotherapy and supportive care, resulting in a larger demand for CIN therapies through hospital pharmacists than through other distribution channels, such as retail or internet pharmacies.
Retail pharmacies have the highest compound annual growth rate (CAGR) in the Chemotherapy-Induced Neutropenia (CIN) Treatment Market due to a variety of variables. These include an increasing trend toward outpatient cancer care and the establishment of retail pharmacy networks, which will make CIN treatments more accessible to patients outside of hospital settings. Furthermore, changes in healthcare policies and insurance coverage may push patients to seek CIN therapies from retail pharmacies, contributing to the channel's rapid market expansion.
Regional Analysis
North America dominated the global chemotherapy-induced neutropenia treatment market in 2023, accounting for 38.2%. The area is predicted to continue to develop at a 3.6% CAGR and maintain its dominance by 2033. The rising prevalence of cancer cases and extensive use of chemotherapy are likely to be the primary growth drivers for the North American chemotherapy-induced neutropenia treatment market. According to MMR research, in 2022, the United States is anticipated to have 1,918,030 new cancer cases and 609,360 cancer deaths, with lung cancer accounting for approximately 350 deaths per day.
Competitive Analysis
Leading competitors in the Chemotherapy-Induced Neutropenia (CIN) Treatment Market are prioritizing strategic measures to preserve their competitive advantage. These include product development and innovation to introduce new medicines and formulations for neutropenia treatment. In addition, corporations form partnerships and collaborate with healthcare institutions and research groups to broaden their market development and improve treatment outcomes. Furthermore, efforts are being made to expand the market into new nations and strengthen distribution networks so that CIN treatments can be made available to a larger patient population.
Recent Developments
March 2023: Coherus BioSciences, Inc. stated that the U.S. Food and Drug Administration ("FDA") has approved UDENYCA (pegfilgrastim-cbqv), a biosimilar pegfilgrastim given the day following chemotherapy to lower the risk of infection as indicated by febrile neutropenia. The UDENYCA autoinjector, with its simple, user-friendly design, is useful in both office and home care settings.
November 2023:Ryzneuta (Efbemalenograstim alfa), a unique long-acting Granulocyte colony-stimulating factor (G-CSF) indicated to reduce the incidence of infection, as manifested by febrile neutropenia, in adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia, has been approved by the United States Food and Drug Administration (FDA).
Key Market Players in the Chemotherapy-Induced Neutropenia Treatment Market
o Amgen Inc.
o BeyondSpring Inc.
o Biocon Biologics Inc.
o Cellerant Therapeutics
o Coherus BioSciences Inc.
o Kyowa Kirin Co. Ltd.
o Novartis AG
o Pfizer Inc.
o Sanofi
o Other Key Players
|
Report Features |
Description |
|
Market Size 2023 |
USD 0.5 Billion |
|
Market Size 2033 |
USD 0.6 Billion |
|
Compound Annual Growth Rate (CAGR) |
3.4% (2023-2033) |
|
Base Year |
2023 |
|
Market Forecast Period |
2024-2033 |
|
Historical Data |
2019-2022 |
|
Market Forecast Units |
Value (USD Billion) |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
By Treatment Type, Distribution Channel, and Region |
|
Geographies Covered |
North America, Europe, Asia Pacific, and the Rest of the World |
|
Countries Covered |
The U.S., Canada, Germany, France, U.K, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil |
|
Key Companies Profiled |
Amgen Inc., BeyondSpring Inc., Biocon Biologics Inc., Cellerant Therapeutics, Coherus BioSciences Inc., Evive Biotech, Kyowa Kirin Co., Ltd., Novartis AG, Pfizer Inc., Sanofi, Other Key Players |
|
Key Market Opportunities |
Increasing Focus on Personalized Medicine |
|
Key Market Dynamics |
Increased Awareness and Early Diagnosis |
📘 Frequently Asked Questions
1. What is the growth rate of the Chemotherapy-Induced Neutropenia Treatment Market?
Answer: Chemotherapy-Induced Neutropenia Treatment Market is growing at a CAGR of 3.4% during the forecast period, from 2023 to 2033.
2. Who are the key players in the Chemotherapy-Induced Neutropenia Treatment Market?
Answer: Amgen Inc., BeyondSpring Inc., Biocon Biologics Inc., Cellerant Therapeutics, Coherus BioSciences Inc., Evive Biotech, Kyowa Kirin Co., Ltd., Novartis AG, Pfizer Inc., Sanofi, Other Key Players
3. How much is the Chemotherapy-Induced Neutropenia Treatment Market in 2023?
Answer: The Chemotherapy-Induced Neutropenia Treatment Market size was valued at USD 0.5 Billion in 2023.
4. What would be the forecast period in the Chemotherapy-Induced Neutropenia Treatment Market?
Answer: The forecast period in the Chemotherapy-Induced Neutropenia Treatment Market report is 2024-2033.

🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
- Accurate & Verified Data:Our insights are trusted by global brands and Fortune 500 companies.
- Complete Transparency:No hidden fees, locked content, or misleading claims — ever.
- 24/7 Analyst Support:Our expert team is always available to help you make smarter decisions.
- Instant Savings:Enjoy a flat $1000 OFF on every report.
- Fast & Reliable Delivery:Get your report delivered within 5 working days, guaranteed.
- Tailored Insights:Customized research that fits your industry and specific goals.



