
🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
At Infinity Market Research, we dont just deliver data — we deliver clarity, confidence, and competitive edge.
In a world driven by insights, we help businesses unlock the infinite potential of informed decisions.
Here why global brands, startups, and decision-makers choose us:
Industry-Centric Expertise
With deep domain knowledge across sectors — from healthcare and technology to manufacturing and consumer goods — our team delivers insights that matter.
Custom Research, Not Cookie-Cutter Reports
Every business is unique, and so are its challenges. Thats why we tailor our research to your specific goals, offering solutions that are actionable, relevant, and reliable.
Data You Can Trust
Our research methodology is rigorous, transparent, and validated at every step. We believe in delivering not just numbers, but numbers that drive real impact.
Client-Centric Approach
Your success is our priority. From first contact to final delivery, our team is responsive, collaborative, and committed to your goals — because you re more than a client; you re a partner.
Recent Reports
Obesity Management Market
GLP-1 Receptor Agonist Market
Clinical Trial Support Services Market
Clinical Trial Support Services Market (By Service (Clinical Trial Site Management, Patient Recruitment Management, Patient recruitment & registry services, Patient retention, Others, Data Management, Administrative staff, IRB, Other Services), By Phase (Phase I, Phase II, Phase III, Phase IV, Other Phase), By Sponsor (Pharmaceutical & Biopharmaceutical, Medical Devices, Other Sponsor), By Indication (Autoimmune or Inflammation, Cardiovascular, Diabetes, Oncology, Other Indication), By Region and Companies)
Aug 2024
Healthcare
Pages: 138
ID: IMR1212
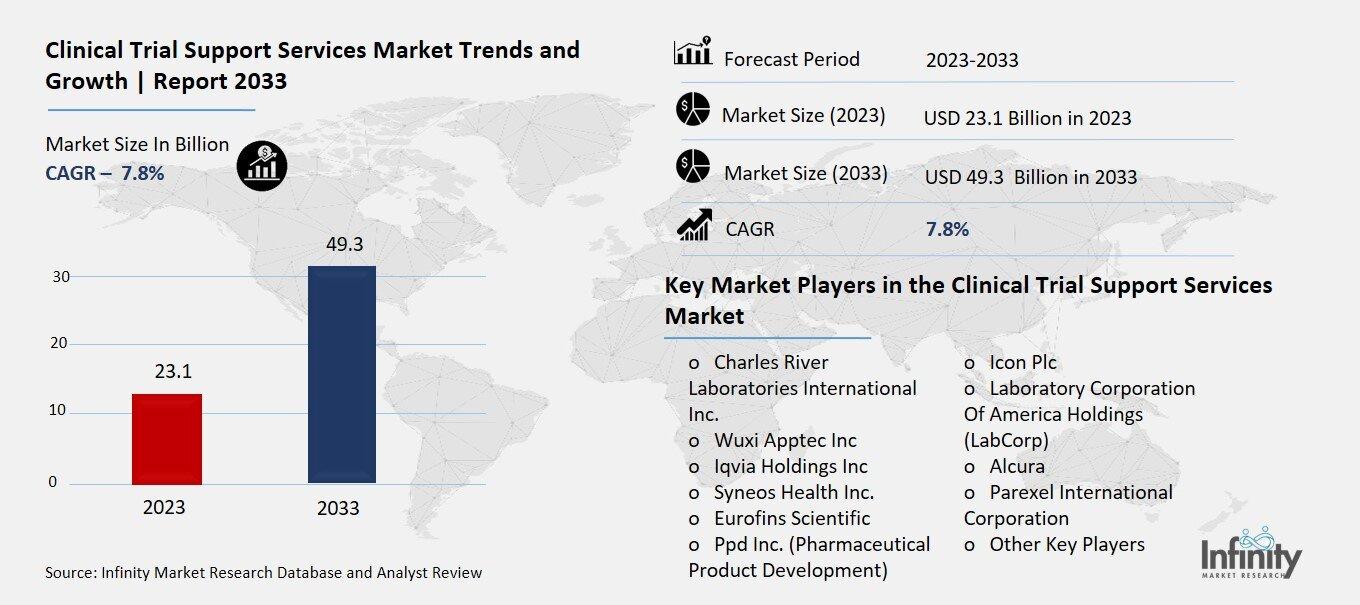
Clinical Trial Support Services Market Overview
Global Clinical Trial Support Services Market size is expected to be worth around USD 49.3 Billion by 2033 from USD 23.1 Billion in 2023, growing at a CAGR of 7.8% during the forecast period from 2023 to 2033.
The Clinical Trial Support Services Market involves businesses and organizations that provide essential services to help pharmaceutical companies, biotech firms, and research institutions conduct clinical trials efficiently. Clinical trials are research studies that test new drugs, medical devices, or treatments to ensure they are safe and effective for humans. These support services include managing patient recruitment, data collection, regulatory compliance, logistics, and monitoring the progress of trials. Companies in this market offer specialized expertise to handle the complexities of clinical research, allowing researchers to focus more on the science rather than the logistics.
This market is growing rapidly because clinical trials are critical in developing new medicines and therapies. As healthcare advances and new treatments are constantly being developed, the demand for efficient clinical trial processes is increasing. The services provided range from initial trial design to managing the end-to-end process, ensuring trials run smoothly, meet regulatory standards, and achieve accurate results. The growth of this market is driven by the increasing complexity of trials, globalization of research, and the need for faster drug development timelines.
Drivers for the Clinical Trial Support Services Market
Rising Demand for Innovative Therapies
The clinical trial support services market is significantly driven by the increasing demand for innovative therapies. As medical science progresses, there is a constant need for new and more effective treatments for various health conditions. Pharmaceutical companies and biotech firms are investing heavily in research and development (R&D) to discover breakthrough therapies. This surge in R&D activities fuels the need for comprehensive support services, including patient recruitment, data management, and regulatory compliance, which are crucial for conducting successful clinical trials. The drive for innovation creates a robust demand for clinical trial support services to ensure that new therapies are tested effectively and meet regulatory standards.
Complexity of Modern Clinical Trials
The complexity of modern clinical trials is another major driver for the growth of clinical trial support services. As clinical trials become more sophisticated, involving advanced technologies, complex protocols, and diverse patient populations, there is a greater need for specialized support. Services such as patient monitoring, data management, and logistics need to be carefully managed to handle the intricate details of these trials. The rise in trials involving complex treatments, including biologics and gene therapies, further increases the demand for professional support services to ensure that all aspects of the trial are managed efficiently and accurately.
Regulatory and Compliance Requirements
Regulatory and compliance requirements are increasingly stringent, driving the need for expert support in clinical trials. Regulatory bodies like the FDA, EMA, and others require detailed documentation, rigorous testing, and adherence to specific guidelines to ensure the safety and efficacy of new treatments. Clinical trial support services help companies navigate these complex regulations, ensuring that trials are conducted in compliance with all legal and ethical standards. This includes preparing regulatory submissions, conducting audits, and ensuring that all trial procedures meet the necessary guidelines, which is essential for the approval of new therapies.
Growth in Global Clinical Trials
The globalization of clinical trials is a significant driver for the clinical trial support services market. As pharmaceutical and biotech companies expand their research efforts internationally, they require support services that can manage trials across different countries and regions. This includes handling international patient recruitment, coordinating with local regulatory agencies, and managing diverse data sets from multiple locations. The growth in global clinical trials necessitates support services that are capable of managing the logistical and regulatory challenges associated with conducting trials on a worldwide scale.
Increasing Focus on Patient-Centric Trials
There is a growing focus on patient-centric trials, which emphasizes the importance of patient experience and outcomes in clinical research. This trend is driving the demand for support services that can enhance patient engagement, streamline trial procedures, and improve overall patient satisfaction. Services such as digital health tools, remote monitoring, and personalized communication strategies are becoming essential in modern clinical trials. By addressing patient needs and preferences, clinical trial support services help ensure higher recruitment and retention rates, ultimately leading to more successful trial outcomes.
Technological Advancements
Technological advancements are revolutionizing the clinical trial process, driving the demand for specialized support services. Innovations such as electronic data capture (EDC), wearable health devices, and advanced analytics are transforming how clinical trials are conducted and managed. Clinical trial support services must adapt to these technological changes, integrating new tools and systems to enhance trial efficiency and data accuracy. The adoption of technology in clinical trials facilitates better data management, real-time monitoring, and streamlined operations, which are crucial for the success of modern clinical research.
Restraints for the Clinical Trial Support Services Market
High Costs of Clinical Trials
One of the main restraints on the clinical trial support services market is the high cost associated with conducting clinical trials. Clinical trials are expensive due to the need for specialized facilities, advanced technology, and a team of professionals to manage various aspects of the trial. The costs of recruiting and compensating participants, conducting extensive testing, and complying with regulatory requirements can be substantial. These high expenses can limit the number of trials conducted and affect the budget allocation for support services, potentially restraining market growth.
Regulatory and Compliance Challenges
Regulatory and compliance challenges are significant obstacles for the clinical trial support services market. Navigating the complex and often varying regulations across different countries can be daunting. Ensuring that all aspects of a clinical trial meet stringent regulatory requirements requires substantial expertise and resources. The risk of non-compliance can lead to delays, increased costs, or even trial rejection, creating a barrier for companies in the clinical trial support services industry. Adapting to constantly changing regulations adds another layer of complexity to managing clinical trials.
Recruitment and Retention Difficulties
Recruitment and retention of trial participants present ongoing challenges in the clinical trial support services market. Finding suitable candidates who meet the specific criteria for a trial can be difficult, especially for rare diseases or specialized conditions. Additionally, retaining participants throughout the trial can be challenging due to the length and intensity of some studies. Recruitment and retention issues can lead to delays and increased costs, impacting the efficiency and effectiveness of clinical trials and posing a restraint on the market.
Data Management and Integrity Issues
Data management and integrity are critical concerns for the clinical trial support services market. Ensuring accurate and reliable data collection and analysis is essential for the success of clinical trials. However, managing large volumes of data, especially from multicenter or global trials, can be complex and prone to errors. Issues such as data discrepancies, incomplete data, or breaches of data security can undermine the credibility of trial results. Maintaining data integrity while managing and analyzing trial data requires robust systems and rigorous quality control measures, which can be resource-intensive.
Technological Limitations
Technological limitations also pose a restraint for the clinical trial support services market. While advancements in technology have improved many aspects of clinical trials, there are still challenges related to the integration and use of new technologies. Not all trial sites may have access to the latest technology or the necessary infrastructure to support advanced tools, such as electronic data capture systems or remote monitoring devices. These limitations can hinder the implementation of innovative solutions and affect the overall efficiency of clinical trial support services.
Operational Complexity
The operational complexity of managing clinical trials adds to the restraints faced by the market. Clinical trials often involve numerous stakeholders, including sponsors, contract research organizations (CROs), investigators, and regulatory bodies. Coordinating among these various parties and managing the different aspects of a trial—such as site selection, monitoring, and reporting—requires a high level of organization and expertise. The operational complexity can lead to inefficiencies and delays, impacting the ability of support services to deliver timely and effective assistance for clinical trials.
Opportunity in the Clinical Trial Support Services Market
Growing Demand for Personalized Medicine
The clinical trial support services market is experiencing significant opportunities due to the growing demand for personalized medicine. As healthcare shifts towards treatments tailored to individual patients based on their genetic, environmental, and lifestyle factors, there is a need for specialized clinical trials to test these personalized therapies. Support services that focus on patient stratification, genetic analysis, and tailored trial designs are essential to accommodate this trend. The rise in personalized medicine drives demand for advanced support services to ensure that these complex trials are conducted efficiently and yield accurate results.
Expansion of Global Clinical Trials
The expansion of global clinical trials presents a major opportunity for the clinical trial support services market. As pharmaceutical and biotechnology companies look to conduct trials across multiple countries to access diverse patient populations and accelerate the development of new therapies, the need for global coordination and management increases. Support services that offer expertise in international regulations, multilingual patient recruitment, and cross-border logistics are crucial for managing these large-scale, multinational trials. This global expansion opens up new markets and revenue streams for support service providers.
Advancements in Technology
Advancements in technology are creating numerous opportunities for the clinical trial support services market. Innovations such as electronic data capture (EDC), remote monitoring tools, and artificial intelligence (AI) are transforming how clinical trials are conducted and managed. Support services that integrate these technologies can enhance data accuracy, streamline trial processes, and improve patient engagement. The adoption of cutting-edge technologies provides opportunities for support services to offer more efficient, high-quality solutions, driving growth in the market.
Increased Focus on Rare Diseases
The increased focus on rare diseases offers substantial opportunities for clinical trial support services. With a growing number of pharmaceutical companies investing in treatments for rare and orphan diseases, there is a need for specialized support services to manage these unique trials. Rare disease trials often require targeted patient recruitment, specialized monitoring, and expert regulatory knowledge. Support services that cater to these specific needs can capitalize on this niche market and offer tailored solutions for rare disease research.
Rising Investments in Biopharmaceuticals
The rise in investments in biopharmaceuticals is another key opportunity for the clinical trial support services market. Biopharmaceutical companies are increasingly focusing on developing biologics, gene therapies, and advanced cell therapies. These complex treatments require extensive and specialized clinical trials. Support services that provide expertise in managing these advanced trials, including handling biological samples, implementing complex protocols, and ensuring compliance with stringent regulations, stand to benefit from the growing biopharmaceutical sector.
Growing Emphasis on Patient-Centric Approaches
There is a growing emphasis on patient-centric approaches in clinical trials, creating new opportunities for support services. Patient-centric trials focus on improving the patient experience by incorporating patient feedback, enhancing convenience, and addressing patient needs throughout the trial process. Support services that offer solutions such as digital health tools, patient engagement platforms, and streamlined trial procedures can play a crucial role in implementing these patient-centered strategies. This focus on patient satisfaction and engagement opens up new avenues for support service providers to enhance their offerings and contribute to more successful trial outcomes.
Trends for the Clinical Trial Support Services Market
Integration of Advanced Technologies
A major trend in the clinical trial support services market is the integration of advanced technologies. Innovations such as electronic data capture (EDC), artificial intelligence (AI), and blockchain are revolutionizing how clinical trials are conducted. EDC systems streamline data collection and management, AI enhances data analysis and predictive modeling, and blockchain ensures data security and integrity. These technologies improve the efficiency and accuracy of trials, leading to faster results and reduced costs. Support services that adopt and integrate these technologies can offer more sophisticated and reliable solutions, keeping pace with the evolving landscape of clinical research.
Rise of Decentralized Clinical Trials
Decentralized clinical trials (DCTs) are becoming increasingly popular, driven by advancements in digital health technologies and the need for more flexible trial designs. DCTs use digital tools, such as mobile apps and wearable devices, to collect data and monitor participants remotely. This approach reduces the need for participants to visit trial sites, making it easier for people to participate and potentially increasing recruitment and retention rates. Support services are adapting to this trend by providing remote monitoring solutions, telemedicine support, and digital data management tools, facilitating the growth of decentralized trials.
Increased Focus on Patient-Centricity
There is a growing trend towards patient-centricity in clinical trials, emphasizing the importance of improving the patient experience. Patient-centric trials prioritize patient needs and preferences, aiming to make participation easier and more engaging. This trend involves incorporating feedback from patients, enhancing communication, and offering flexible participation options. Support services are increasingly focusing on developing solutions that address patient concerns and streamline trial procedures, such as providing mobile apps for patient engagement and remote monitoring tools to reduce the burden on participants.
Expansion of Global Trials
The expansion of clinical trials into new and diverse global markets is another significant trend. As pharmaceutical companies seek to reach broader patient populations and accelerate drug development, there is a growing need for support services that can manage international trials. This includes navigating varying regulatory requirements, coordinating with local trial sites, and managing multilingual and culturally diverse patient populations. Support services that offer expertise in global trial management and local regulatory compliance are well-positioned to capitalize on this trend and provide essential services for multinational studies.
Emphasis on Data Privacy and Security
With the increasing amount of sensitive data being collected in clinical trials, there is a heightened emphasis on data privacy and security. Regulations such as the General Data Protection Regulation (GDPR) and the Health Insurance Portability and Accountability Act (HIPAA) mandate strict standards for data protection. Clinical trial support services are focusing on implementing robust data security measures to protect patient information and ensure compliance with these regulations. This includes employing advanced encryption technologies, secure data storage solutions, and regular audits to safeguard data integrity and maintain trust in the research process.
Growth in Early-Phase Clinical Trials
There is a noticeable trend toward increasing the number of early-phase clinical trials, driven by the need to test new therapies and innovative treatments at the initial stages of development. Early-phase trials, including Phase I and Phase II studies, are crucial for assessing the safety and efficacy of new drugs. Support services are responding to this trend by offering specialized services for early-phase trials, such as expert patient recruitment strategies, intensive monitoring, and detailed data analysis. This focus on early-phase trials reflects the industry's drive to accelerate drug development and bring new treatments to market more efficiently.
Segments Covered in the Report
By Service
o Clinical Trial Site Management
o Patient Recruitment Management
o Patient recruitment & registry services
o Patient retention
o Others
o Data Management
o Administrative staff
o IRB
o Other Service
By Phase
o Phase I
o Phase II
o Phase III
o Phase IV
o Other Phase
By Sponsor
o Pharmaceutical & Biopharmaceutical
o Medical Devices
o Other Sponsor
By Indication
o Autoimmune or Inflammation
o Cardiovascular
o Diabetes
o Oncology
o Other Indication
Segment Analysis
By Service Analysis
Clinical trial site administration entails recruitment, retention, and monitoring. It held the greatest market share of 43.8% in 2023, with a CAGR of 8.9%. The rising number of clinical trials, the high frequency of chronic diseases, and the growing number of CROs offering services are all expected to drive the market in the future years. Site monitoring accounts for 9-14%, whereas site retention accounts for 9-16%. Thus, clinical trial site management contributes between 29% and 59% of the total cost of a trial.
Proper site management by the sponsor and/or CRO is critical to trial execution and success. A sufficient level of site administration and monitoring allows facilities to efficiently recruit, treat, and retain subjects while ensuring regulatory compliance, protocol adherence, subject rights protection, subject safety, and overall management of screened and recruited subjects.
By Phase Analysis
In 2023, the phase III segment led the market, accounting for 55.6%. This increase can be linked to the fact that phase III clinical studies are the costliest and involve a large number of participants. The failure rate in this phase is the highest due to the sample size, and the study design necessitates sophisticated dosing at an optimal level. Failure results in both human and financial losses, with the majority of failures caused by noncompliance with safety and efficacy criteria.
The phase I category is expected to have the fastest CAGR (9.0%) during the projection period. Phase I clinical trial support services include sample collection management, early-phase patient screening, data management, and assay redesign, among others. The United States, Europe, and China followed by Canada and Australia, are thus acknowledged as critical locations for registering and conducting large-scale phase I clinical studies. As a result, clinical trial management services are in high demand in these countries.
By Sponsor Analysis
In 2023, the pharmaceutical and biopharmaceutical firms' sponsor sector led the market, accounting for more than 71.8% of total revenue. The segment is also expected to have the quickest CAGR from 2023 to 2033. This is largely due to increased R&D spending and the release of new pharmaceuticals over the last two decades. In 2019, the pharmaceutical sector spent USD 83 billion on R&D.
The medical device business segment is expected to increase at a CAGR of 6.8% over the forecast period. Medical device makers are minor customers in the clinical research market, with clinical research facilities focusing on the more profitable industries of biotechnology, pharmaceuticals, and fundamental research. However, as the FDA places a greater emphasis on excellent clinical procedures, device manufacturers will interface with this complex industry on a far more frequent basis.
By Indication Analysis
Clinical trial support services are crucial for effectively managing trials across various medical conditions. For autoimmune or inflammatory conditions like rheumatoid arthritis, lupus, and multiple sclerosis, these services focus on recruiting patients with specific diseases, monitoring their complex health needs, and managing side effects from treatments that suppress the immune system. They also include advanced data management systems to track disease progression and treatment responses. In cardiovascular trials, which test treatments for heart and blood vessel diseases such as hypertension and heart failure, support services handle large-scale patient recruitment, perform regular cardiovascular assessments, and analyze data on heart function and blood pressure to ensure the safety and effectiveness of new therapies.
Similarly, diabetes-related trials, addressing type 1 and type 2 diabetes, require specialized support for patient recruitment, frequent blood glucose monitoring, and coordination with endocrinologists for comprehensive care. In oncology trials, aimed at developing new cancer treatments, support services manage patient enrollment, coordinate w0069th oncologists, and oversee extensive testing and monitoring. Robust data management is essential to track tumor responses and patient outcomes. Each of these trial types benefits from tailored support services that address the unique challenges of their respective conditions, ensuring thorough and effective evaluation of new treatments.
Regional Analysis
North America dominated the global market in 2023, accounting for 51.7% of the total since the bulk of pharmaceutical companies in the United States conduct their operations in this region. This industry is expected to expand due to the large number of clinical studies taking place in the region. Major R&D investments and government funding for clinical trials are driving market growth. The existence of prominent CROs providing clinical trial support services, as well as global pharmaceutical and biotech companies investing heavily in clinical research, has helped to drive market expansion in North America.
Asia Pacific is expected to be the fastest-growing regional market, with a CAGR of 8.5% over the projected period. Factors propelling the APAC market include a growing patient population, ease of administrative compliance, low study costs, and the presence of a few top clinical organizations at sites.
Competitive Analysis
Leading market players are investing substantially in R&D to enhance their service offerings, which will help the viral clearance market grow even more. Market participants are also engaging in several strategic initiatives to grow their worldwide footprint, with significant market developments including new service launches, contractual agreements, mergers and acquisitions, increased investments, and collaboration with other organizations. To grow and thrive in a more competitive and expanding market, the viral clearance business must provide cost-effective products.
Recent Developments
In November 2022: Amgen released end-of-treatment data from their Phase 2 OCEAN(a)-DOSE study of experimental olpasiran (previously AMG 890) in adults. Olpasiran was observed to lower lipoprotein(a) levels by more than 95% in patients with established atherosclerotic cardiovascular disease.
In November 2022: AstraZeneca reported favorable results from two Phase III trials in breast cancer and uncommon blood disorders, as well as positive topline results from a Phase III trial in ophthalmology.
Key Market Players in the Clinical Trial Support Services Market
o Charles River Laboratories International Inc.
o Ppd Inc. (Pharmaceutical Product Development)
o Icon Plc
o Laboratory Corporation Of America Holdings (LabCorp)
o Alcura
o Parexel International Corporation
o Other Key Players
|
Report Features |
Description |
|
Market Size 2023 |
USD 23.1 Billion |
|
Market Size 2033 |
USD 49.3 Billion |
|
Compound Annual Growth Rate (CAGR) |
7.8% (2023-2033) |
|
Base Year |
2023 |
|
Market Forecast Period |
2024-2033 |
|
Historical Data |
2019-2022 |
|
Market Forecast Units |
Value (USD Billion) |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
By Service, Phase, Sponsor, Indication, and Region |
|
Geographies Covered |
North America, Europe, Asia Pacific, and the Rest of the World |
|
Countries Covered |
The U.S., Canada, Germany, France, U.K, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil |
|
Key Companies Profiled |
Charles River Laboratories International, Inc., Wuxi Apptec, Inc, Iqvia Holdings, Inc, Syneos Health, Inc., Eurofins Scientific, Ppd, Inc. (Pharmaceutical Product Development), Icon Plc, Laboratory Corporation Of America Holdings (Labcorp), Alcura, Parexel International Corporation, Other Key Players |
|
Key Market Opportunities |
Rising Investments in Biopharmaceuticals |
|
Key Market Dynamics |
Increasing Focus on Patient-Centric Trials |
📘 Frequently Asked Questions
1. What is the growth rate of the Clinical Trial Support Services Market?
Answer: Clinical Trial Support Services Market is growing at a CAGR of 7.8% during the forecast period, from 2023 to 2033.
2. Who are the key players in the Clinical Trial Support Services Market?
Answer: Charles River Laboratories International, Inc., Wuxi Apptec, Inc, Iqvia Holdings, Inc, Syneos Health, Inc., Eurofins Scientific, Ppd, Inc. (Pharmaceutical Product Development), Icon Plc, Laboratory Corporation Of America Holdings (Labcorp), Alcura, Parexel International Corporation, Other Key Players
3. How much is the Clinical Trial Support Services Market in 2023?
Answer: The Clinical Trial Support Services Market size was valued at USD 23.1 Billion in 2023.
4. What would be the forecast period in the Clinical Trial Support Services Market?
Answer: The forecast period in the Clinical Trial Support Services Market report is 2024-2033.

🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
- Accurate & Verified Data:Our insights are trusted by global brands and Fortune 500 companies.
- Complete Transparency:No hidden fees, locked content, or misleading claims — ever.
- 24/7 Analyst Support:Our expert team is always available to help you make smarter decisions.
- Instant Savings:Enjoy a flat $1000 OFF on every report.
- Fast & Reliable Delivery:Get your report delivered within 5 working days, guaranteed.
- Tailored Insights:Customized research that fits your industry and specific goals.



