
🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
At Infinity Market Research, we dont just deliver data — we deliver clarity, confidence, and competitive edge.
In a world driven by insights, we help businesses unlock the infinite potential of informed decisions.
Here why global brands, startups, and decision-makers choose us:
Industry-Centric Expertise
With deep domain knowledge across sectors — from healthcare and technology to manufacturing and consumer goods — our team delivers insights that matter.
Custom Research, Not Cookie-Cutter Reports
Every business is unique, and so are its challenges. Thats why we tailor our research to your specific goals, offering solutions that are actionable, relevant, and reliable.
Data You Can Trust
Our research methodology is rigorous, transparent, and validated at every step. We believe in delivering not just numbers, but numbers that drive real impact.
Client-Centric Approach
Your success is our priority. From first contact to final delivery, our team is responsive, collaborative, and committed to your goals — because you re more than a client; you re a partner.
Recent Reports
Obesity Management Market
GLP-1 Receptor Agonist Market
Companion Diagnostics Market
Companion Diagnostics Market Global Industry Analysis and Forecast (2024-2033) by Technology (Polymerase Chain Reaction, Immunohistochemistry, In-situ Hybridization, Next Generation Gene Sequencing, and Other Technologies), Indication (Neurological Diseases, Cancer, Infectious Diseases, and Other Indications), End-User (Pharmaceutical & Biopharmaceutical Companies, Contract Research Organizations (CROs), and Reference Laboratories) and Region
Jun 2025
Healthcare
Pages: 138
ID: IMR2055
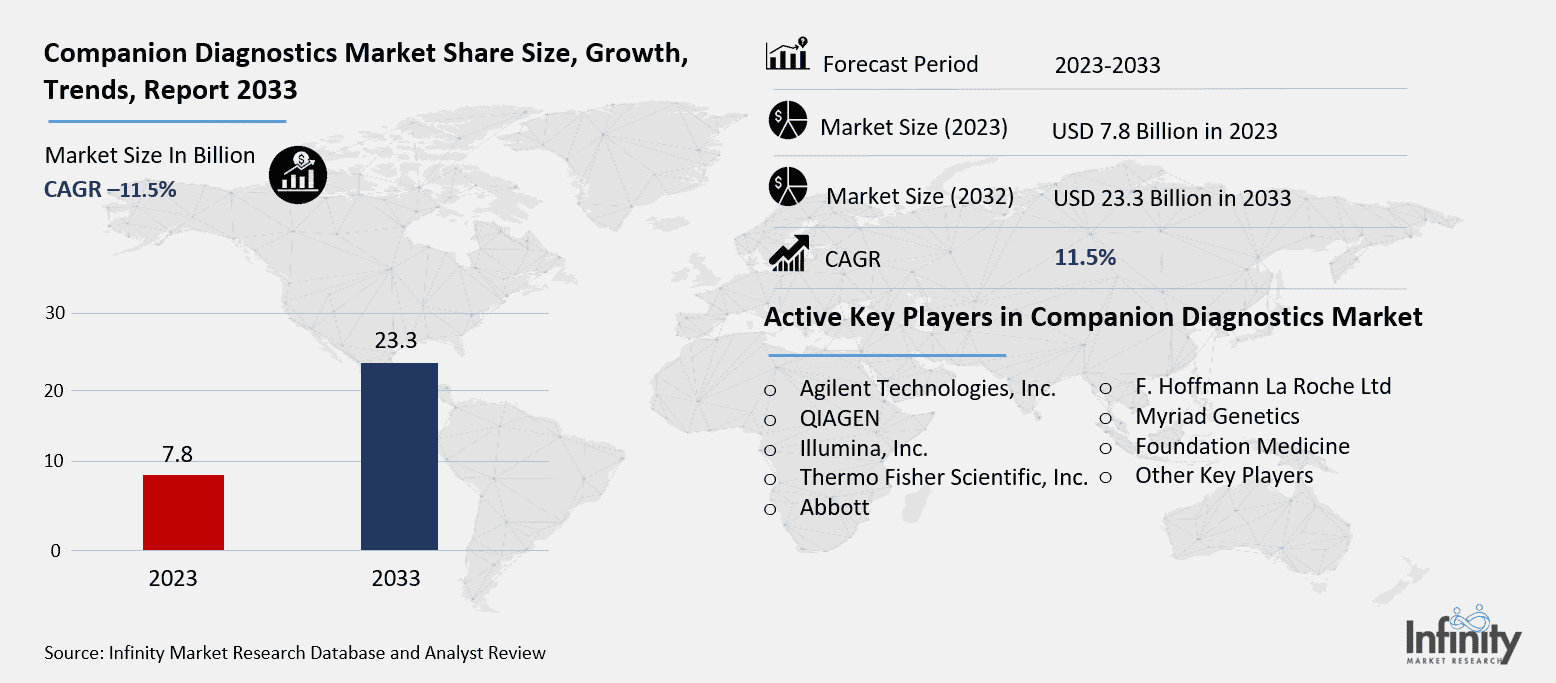
Companion Diagnostics Market Synopsis
The Global Companion Diagnostics Market was valued at USD 7.8 billion in 2023 and is expected to grow from USD 8.7 billion in 2024 to USD 23.3 billion by 2033, reflecting a CAGR of 11.5% over the forecast period.
The companion diagnostics market consists of the healthcare products that check whether a patient can take a particular medicine or treatment. By using these tests, it becomes possible to decide on the best treatment for each person considering their genes or health markers. There is a noticeable rise in the market because more patients in oncology and chronic conditions now use targeted therapies, people know more about personalized treatments, and regulators support the simultaneous development of drugs and diagnostics.
Companion Diagnostics Market Driver Analysis
Increased Prevalence of Cancer and Chronic Diseases
Cancer, cardiovascular, and autoimmune diseases are causing a big increase in companion diagnostics sales. Such serious conditions can only be treated well when accurate and specialized plans are used to achieve the best results and limit the risks. With companion diagnostics, healthcare providers can spot certain changes in a patient’s DNA that indicate a disease, so they know which drugs to use. In cancer care, this kind of testing is vital to see if a patient can use a particular medication or not. In addition, when dealing with cardiovascular and autoimmune problems, companion diagnostics help to choose appropriate biologic and other specialized drugs.
Companion Diagnostics Market Restraint Analysis
Regulatory and Reimbursement Challenges
The growth of this market is badly affected by strict approval procedures and not enough insurance availability in particular places. Approval for both the companion diagnostic and the therapeutic drug must often be given nearly at the same time. Since there is a long process of clinical approval, this type of drug development takes a lot of time and money. Besides, many underdeveloped or financially strained hospitals do not receive generous insurance payments for these highly advanced science-based tests. Since this topic is not widely discussed, people are not likely to use them as much.
Companion Diagnostics Market Opportunity Analysis
Development of Point-of-Care Testing
Rapid diagnostic tools designed to be portable are being considered for wider use in companion diagnostics due to their speed and accuracy. With these tools, specialists can perform tests without relying on big laboratories, so people in remote or strained places have better access to diagnosis. With lab results coming fast, doctors are able to decide on the right treatments for patient’s sooner. Because healthcare is becoming more patient centered, medical companies are counting on portable diagnostics for cancer and infectious diseases.
Companion Diagnostics Market Trend Analysis
Growing Use of Companion Diagnostics in Clinical Trials
Improving how patients are grouped and enhancing the process of developing drugs supports the use of companion diagnostics in medical research. Using particular biomarkers, companion diagnostics help doctors evaluate patients and predict which treatments would be effective for them. Targeting certain patients in this way improves results and shortens how much time each trial takes. Also, it ensures that patients with no reactions are not given useless medicines, creating a safer and more ethical way to medicate people. In addition, by addressing specific groups of patients, pharmaceutical companies can use resources more efficiently and speed up the process of bringing new treatments to people.
Companion Diagnostics Market Segment Analysis:
The Companion Diagnostics Market is segmented on the basis of Technology, Indication, and End-User.
By Technology
o Polymerase Chain Reaction
o Immunohistochemistry
o In-situ Hybridization
o Next Generation Gene Sequencing
o Other Technologies
By Indication
o Neurological Diseases
o Cancer
o Infectious Diseases
o Other Indications
By End-User
o Pharmaceutical & Biopharmaceutical Companies
o Contract Research Organizations (CROs)
o Reference Laboratories
By Region
o North America (U.S., Canada, Mexico)
o Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe)
o Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe)
o Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC)
o Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa)
o South America (Brazil, Argentina, Rest of SA)
By Technology, Polymerase Chain Reaction Segment is Expected to Dominate the Market During the Forecast Period
Of the technologies discussed in this research study, the polymerase chain reaction segment is expected to account for the largest market share of companion diagnostics market in the forecast period. PCR is commonly used and proven to work very well, allowing experts to detect genetic mutations and biomarkers associated with several diseases. Since it is very sensitive, accurate, affordable, and fast, cytogenetics is widely used in hospitals and laboratories. In the context of choosing targeted therapies, PCR is key to figuring out if a patient is eligible, mainly in cases of oncology.
By Indication, the Cancer Segment is Expected to Held the Largest Share
The cancer segment is likely to dominate the market. The main reason behind this dominance is that cancer is on the rise globally, which makes people need treatments that are accurate and protect from serious side effects. Identifying the right genetic mutations, proteins, or biomarkers in cancer using companions helps decide which treatments will be most helpful for certain patients. Many cancers, including breast, lung, colorectal, and melanoma, use these tests because precision medicine is making a big difference in treatment.
By End-User, the Pharmaceutical & Biopharmaceutical Companies Segment is Expected to Held the Largest Share
By end-user, the pharmaceutical and biopharmaceutical companies segment is expected to hold the largest share of the companion diagnostics market during the forecast period. The process of developing and selling targeted therapies now depends heavily on these firms providing companion diagnostic tests to pick out appropriate patients. Creating effective diagnostics for drugs makes clinical trials more efficient, raises the chances of being approved by authorities, and boosts the results patients receive. Since interest in personalized medicine is rising, drug and biotech firms are putting much effort into combining drug development with diagnostics, often by working with diagnostic companies.
Companion Diagnostics Market Regional Insights:
North America is Expected to Dominate the Market Over the Forecast period
North America is expected to dominate the companion diagnostics market over the forecast period. The presence of up-to-date health facilities, solid rules, and substantial research and development spending is what drives this leadership in the industry. Many well-known pharmaceutical and biotech firms based in the region are focused on inventing targeted therapies and companion diagnostic tests. Besides, the fact that healthcare providers and patients are becoming more familiar and accepting of personalized medicine is increasing the demand for the market. Various government and insurance company decisions in favor of telehealth, as well as new technologies, keep North America as the main market leader. Out of all the countries in the region, the United States is the biggest because of its advanced clinic trial program and administrations.
Recent Development
· In January 2025, Roche announced that the FDA has approved a label expansion for the PATHWAY anti-Rabbit Monoclonal Primary Antibody. This update allows for the identification of patients with HR-positive, HER2-ultralow metastatic breast cancer who may be eligible for treatment.
· In April 2024, Labcorp, a global leader in innovative laboratory services, announced that the FDA has approved its nAbCte Anti-AAVRh74var HB-FE Assay. This companion diagnostic (CDx) is designed to evaluate patient eligibility for treatment with BEQVZ™, Pfizer’s newly FDA-approved gene therapy for hemophilia B.
Active Key Players in the Companion Diagnostics Market
o Agilent Technologies, Inc.
o QIAGEN
o Illumina, Inc.
o Thermo Fisher Scientific, Inc.
o Abbott
o F. Hoffmann La Roche Ltd
o Myriad Genetics
o Foundation Medicine
o Other Key Players
Global Companion Diagnostics Market Scope:
|
Global Companion Diagnostics Market | |||
|
Base Year: |
2024 |
Forecast Period: |
2024-2033 |
|
Historical Data: |
2017 to 2023 |
Market Size in 2023: |
USD 7.8 Billion |
|
Market Size in 2024: |
USD 8.7 Billion | ||
|
Forecast Period 2024-33 CAGR: |
11.5% |
Market Size in 2033: |
USD 23.3 Billion |
|
Segments Covered: |
By Technology |
· Polymerase Chain Reaction · Immunohistochemistry · In-situ Hybridization · Next Generation Gene Sequencing · Other Technologies | |
|
By Indication |
· Neurological Diseases · Cancer · Infectious Diseases · Other Indications | ||
|
By End-User |
· Pharmaceutical & Biopharmaceutical Companies · Contract Research Organizations (CROs) · Reference Laboratories | ||
|
By Region |
· North America (U.S., Canada, Mexico) · Eastern Europe (Bulgaria, The Czech Republic, Hungary, Poland, Romania, Rest of Eastern Europe) · Western Europe (Germany, UK, France, Netherlands, Italy, Russia, Spain, Rest of Western Europe) · Asia Pacific (China, India, Japan, South Korea, Malaysia, Thailand, Vietnam, The Philippines, Australia, New-Zealand, Rest of APAC) · Middle East & Africa (Turkey, Bahrain, Kuwait, Saudi Arabia, Qatar, UAE, Israel, South Africa) · South America (Brazil, Argentina, Rest of SA) | ||
|
Key Market Drivers: |
· Increased Prevalence of Cancer and Chronic Diseases | ||
|
Key Market Restraints: |
· Regulatory and Reimbursement Challenges | ||
|
Key Opportunities: |
· Development of Point-of-Care Testing | ||
|
Companies Covered in the report: |
· Agilent Technologies, Inc., QIAGEN, Illumina, Inc., Thermo Fisher Scientific, Inc., and Other Key Players. | ||
📘 Frequently Asked Questions
1. What would be the forecast period in the Companion Diagnostics Market Research report?
Answer: The forecast period in the Companion Diagnostics Market Research report is 2024-2033.
2. Who are the key players in the Companion Diagnostics Market?
Answer: Agilent Technologies, Inc., QIAGEN, Illumina, Inc., Thermo Fisher Scientific, Inc., and Other Key Players.
3. What are the segments of the Companion Diagnostics Market?
Answer: The Companion Diagnostics Market is segmented into Technology, Indication, End-User, and Regions. By Technology, the market is categorized into Polymerase Chain Reaction, Immunohistochemistry, In-situ Hybridization, Next Generation Gene Sequencing, and Other Technologies. By Indication, the market is categorized into Neurological Diseases, Cancer, Infectious Diseases, and Other Indications. By End-User, the market is categorized into Pharmaceutical & Biopharmaceutical Companies, Contract Research Organizations (CROs), and Reference Laboratories. By region, it is analyzed across North America (U.S.; Canada; Mexico), Eastern Europe (Bulgaria; The Czech Republic; Hungary; Poland; Romania; Rest of Eastern Europe), Western Europe (Germany; UK; France; Netherlands; Italy; Russia; Spain; Rest of Western Europe), Asia-Pacific (China; India; Japan; Southeast Asia, etc.), South America (Brazil; Argentina, etc.), Middle East & Africa (Saudi Arabia; South Africa, etc.).
4. What is the Companion Diagnostics Market?
Answer: The companion diagnostics market is dedicated to creating and utilizing tests that identify what treatments will be best for each patient in healthcare. Techniques like genomics, proteomics, and molecular biology are often combined with specific drugs to find the patient’s responses.
5. How big is the Companion Diagnostics Market?
Answer: The Global Companion Diagnostics Market was valued at USD 7.8 billion in 2023 and is expected to grow from USD 8.7 billion in 2024 to USD 23.3 billion by 2033, reflecting a CAGR of 11.5% over the forecast period.

🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
- Accurate & Verified Data:Our insights are trusted by global brands and Fortune 500 companies.
- Complete Transparency:No hidden fees, locked content, or misleading claims — ever.
- 24/7 Analyst Support:Our expert team is always available to help you make smarter decisions.
- Instant Savings:Enjoy a flat $1000 OFF on every report.
- Fast & Reliable Delivery:Get your report delivered within 5 working days, guaranteed.
- Tailored Insights:Customized research that fits your industry and specific goals.



