
🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
At Infinity Market Research, we dont just deliver data — we deliver clarity, confidence, and competitive edge.
In a world driven by insights, we help businesses unlock the infinite potential of informed decisions.
Here why global brands, startups, and decision-makers choose us:
Industry-Centric Expertise
With deep domain knowledge across sectors — from healthcare and technology to manufacturing and consumer goods — our team delivers insights that matter.
Custom Research, Not Cookie-Cutter Reports
Every business is unique, and so are its challenges. Thats why we tailor our research to your specific goals, offering solutions that are actionable, relevant, and reliable.
Data You Can Trust
Our research methodology is rigorous, transparent, and validated at every step. We believe in delivering not just numbers, but numbers that drive real impact.
Client-Centric Approach
Your success is our priority. From first contact to final delivery, our team is responsive, collaborative, and committed to your goals — because you re more than a client; you re a partner.
Recent Reports
Obesity Management Market
GLP-1 Receptor Agonist Market
In Vitro Toxicology Testing Market
In Vitro Toxicology Testing Market (By Product (Consumables, Assays, Instruments, Software, Services, Other Product), By Technology (Cell Culture Technology, High Throughput Technology, Molecular Imaging Technology, OMICS Technology, Other Technology), By Method (Cellular Assay, Biochemical Assay, In Silico, Ex-vivo), By End-use (Pharmaceutical Industry, Cosmetics & Household Products, Academic Institutes & Research Laboratories, Diagnostics, Chemicals Industry, Food Industry), By Region and Companies)
Aug 2024
Healthcare
Pages: 138
ID: IMR1206
In Vitro Toxicology Testing Market Overview
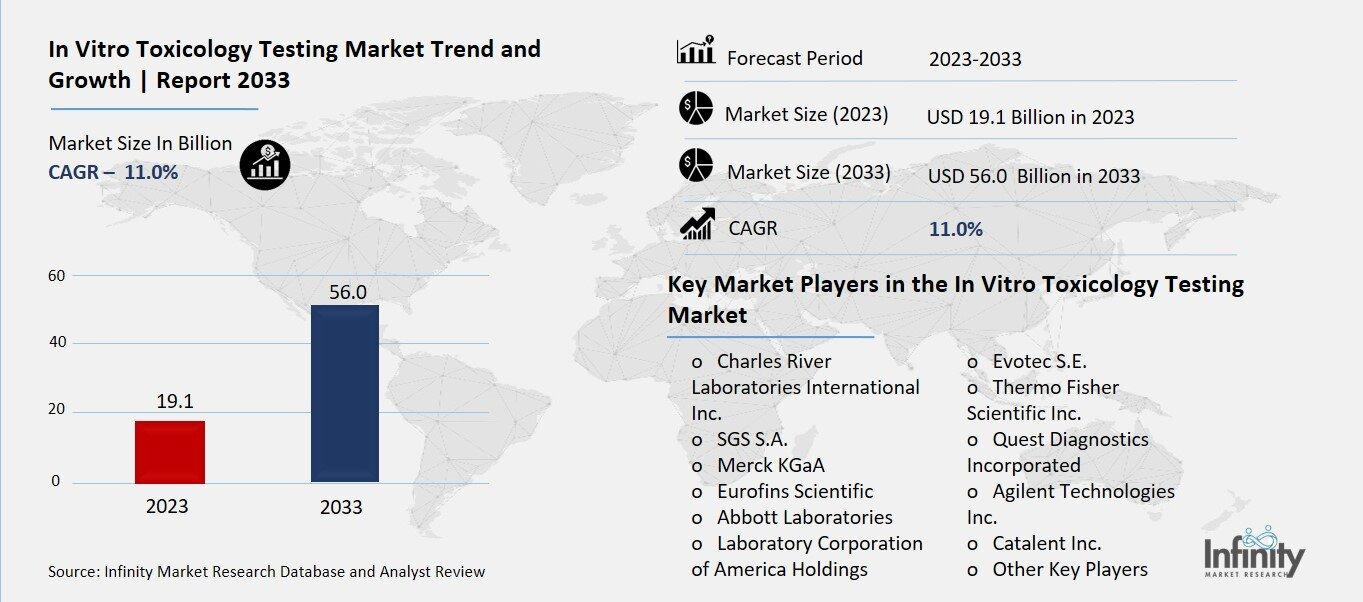
Global In Vitro Toxicology Testing Market size is expected to be worth around USD 56.0 Billion by 2033 from USD 19.1 Billion in 2023, growing at a CAGR of 11.0% during the forecast period from 2023 to 2033.
The In Vitro Toxicology Testing Market is the industry that provides tests to check if substances, like chemicals or drugs, can be harmful. "In vitro" means testing outside a living organism, usually in a lab setting with cells or tissues. Instead of testing on animals or humans, scientists use these lab tests to see how substances might affect living cells. This helps in understanding potential risks before moving on to more extensive testing.
Companies create and sell these lab-based tests in this market to ensure that new products, like medicines or chemicals, are safe. This testing is important for protecting human health and the environment. As more regulations require safety testing and technology advances, this market grows. It's crucial to ensure that new products don’t cause harm before they are used widely.
Drivers for the In Vitro Toxicology Testing Market
Increasing Regulatory Requirements
One major driver for the in vitro toxicology testing market is the rise in regulatory requirements for product safety. Governments and health agencies worldwide are implementing stricter regulations to ensure that chemicals, drugs, and cosmetics are safe for human use. These regulations often mandate thorough testing to detect any potential harmful effects before products are approved for public use. This has led to a growing demand for in vitro tests, as they provide a way to comply with these regulations efficiently and ethically. By using lab-based methods instead of animal testing, companies can meet regulatory standards and avoid potential legal issues or delays in product approvals.
Advances in Technology
Technological advancements are significantly driving the in vitro toxicology testing market. Innovations in cell culture techniques, high-throughput screening, and computer modeling have improved the accuracy and efficiency of toxicology tests. For example, modern cell-based assays can replicate human responses more accurately than older methods, leading to better predictions of a substance’s safety. As technology continues to advance, these tests become more reliable and cost-effective, encouraging more companies to adopt them. This technological progress helps speed up the testing process, reducing costs, and improving the overall quality of results.
Growing Awareness of Animal Welfare
The increasing awareness of animal welfare is another key factor driving the market. Many organizations and consumers are pushing for the reduction of animal testing due to ethical concerns. In vitro testing offers an alternative that avoids using animals while still providing valuable safety data. This shift in public opinion and ethical considerations is leading companies to seek more humane testing methods. As a result, the demand for in vitro toxicology testing is growing, as it aligns with the values of both consumers and regulatory bodies, fostering a more ethical approach to product safety.
Rising Demand for Personal Care Products
The growing demand for personal care and cosmetic products is also driving the in vitro toxicology testing market. As consumers increasingly focus on personal health and wellness, there is a higher demand for new and innovative beauty and skincare products. These products must undergo rigorous safety testing to ensure they are free from harmful effects. In vitro tests are particularly valuable in this sector because they allow for the screening of various ingredients without extensive animal testing. This helps companies quickly bring new products to market while ensuring their safety, thus supporting the overall growth of the market.
Focus on Drug Development and Safety
The focus on drug development and safety is another important driver. Pharmaceutical companies are investing heavily in developing new drugs and treatments. Ensuring these drugs are safe before they reach the market is crucial to avoid potential health risks and costly recalls. In vitro, toxicology testing provides an effective method for evaluating the safety of new drugs early in the development process. This allows for the identification of potential issues before moving to clinical trials, thus reducing the risk of adverse effects in humans and speeding up the drug development timeline.
Increased Research and Development Activities
Finally, increased research and development (R&D) activities in the life sciences sector contribute to the growth of the in vitro toxicology testing market. With ongoing research into new compounds and their effects, there is a constant need for advanced testing methods. Researchers rely on in vitro tests to explore how new substances interact with biological systems. This continuous R&D drives innovation and expansion in the market, as new testing methods and products are developed to meet the evolving needs of the scientific community and industry.
Restraints for the In Vitro Toxicology Testing Market
High Cost of Advanced Technologies
One significant restraint for the in vitro toxicology testing market is the high cost associated with advanced technologies. Modern in vitro testing methods, such as high-throughput screening and sophisticated cell cultures, require expensive equipment and materials. Additionally, maintaining and operating this technology involves ongoing costs for training, calibration, and maintenance. This can be a barrier for smaller companies or laboratories with limited budgets. The initial investment required for these technologies can restrict their adoption and slow down market growth.
Limited Predictive Accuracy
Another challenge is the limited predictive accuracy of some in vitro tests. While in vitro methods are useful, they do not always perfectly replicate the complex interactions within a whole organism. This can lead to discrepancies between lab results and real-world outcomes. For example, some tests might not fully capture how a substance will behave in different tissues or under varying conditions in a living body. This limitation can result in incomplete safety assessments and may require additional testing or validation, complicating the overall process.
Regulatory Acceptance Issues
Regulatory acceptance is a crucial factor affecting the in vitro toxicology testing market. Although there is growing acceptance of these methods, not all regulatory agencies fully embrace them as replacements for traditional animal testing. Some regulations still require animal testing alongside in vitro tests to ensure comprehensive safety evaluation. This can limit the use of in vitro methods and increase the time and cost required for product approval. Companies must navigate complex regulatory requirements, which can be a significant challenge and hinder the market's growth.
Complexity of Standardization
Standardizing in vitro testing methods is another obstacle. The lack of universal standards can lead to variability in test results, making it difficult to compare data across different studies or laboratories. This lack of consistency can undermine the reliability of test results and make it challenging for companies to trust the outcomes. Efforts to develop and implement standardized protocols are ongoing, but until these are widely adopted, variability in testing practices can constrain market development.
Integration with Existing Testing Protocols
Integrating in vitro tests with existing testing protocols poses a challenge. Many industries have established processes that include animal testing, and transitioning to in vitro methods requires adjustments in procedures, training, and data interpretation. This shift can be slow and complex, particularly in industries where traditional methods are deeply ingrained. The need for integration can delay the adoption of in vitro testing and limit its impact on market growth.
Market Resistance from Traditionalists
Finally, resistance from traditionalists within the scientific and regulatory communities can slow the adoption of in vitro testing. Some stakeholders may be hesitant to fully embrace new methods due to a preference for established practices or skepticism about the new technologies' effectiveness. This resistance can delay the acceptance and widespread use of in vitro methods, impacting market expansion and innovation. Efforts to educate and demonstrate the benefits of in vitro testing are essential to overcoming this resistance.
Opportunity in the In Vitro Toxicology Testing Market
Growing Demand for Safer Products
One significant opportunity for the in vitro toxicology testing market is the increasing demand for safer consumer products. As consumers become more aware of health and safety concerns, there is a growing expectation for products to be thoroughly tested before reaching the market. In vitro testing provides a way to assess the safety of chemicals, drugs, and cosmetics without relying heavily on animal testing. This aligns with consumer preferences for humane and reliable testing methods, creating a strong market demand for innovative in vitro solutions that can ensure product safety effectively.
Advancements in Biotechnology
Advancements in biotechnology offer another promising opportunity for the in vitro toxicology testing market. New technologies, such as organ-on-a-chip models and advanced cell cultures, are revolutionizing how toxicology tests are conducted. These innovations allow for more accurate and predictive assessments of how substances will affect human health. As biotechnology continues to evolve, it presents opportunities for the development of more sophisticated and effective in vitro tests. Companies that invest in these cutting-edge technologies can gain a competitive edge and drive market growth.
Expansion of Regulatory Frameworks
The expansion of regulatory frameworks that support in vitro testing is creating additional opportunities. Regulatory agencies are increasingly recognizing the value of in vitro methods as part of comprehensive safety assessments. As more guidelines and policies are developed to incorporate these methods, there will be greater adoption of in vitro testing in various industries. This trend is likely to accelerate as regulatory bodies push for more sustainable and ethical testing practices, offering a favorable environment for the growth of the in vitro toxicology testing market.
Rising Investment in R&D
Increased investment in research and development (R&D) is fueling opportunities in the in vitro toxicology testing market. As pharmaceutical and chemical companies focus on developing new products, there is a growing need for reliable testing methods to ensure safety and efficacy. Investing in R&D for in vitro testing technologies can lead to breakthroughs that enhance the accuracy and efficiency of safety assessments. This investment not only supports the development of new testing solutions but also drives market growth by meeting the evolving needs of the industry.
Integration with Personalized Medicine
The integration of in vitro toxicology testing with personalized medicine is another promising opportunity. Personalized medicine aims to tailor treatments and products to individual genetic profiles, which requires precise and reliable testing methods. In vitro, tests can be adapted to assess how different individuals might respond to substances based on their genetic makeup. This integration enhances the relevance and applicability of toxicology tests, supporting the growth of personalized medicine and expanding the market for in vitro testing.
Emergence of New Markets
Emerging markets in developing regions present significant opportunities for the in vitro toxicology testing market. As these regions experience economic growth and increased industrial activity, there is a rising need for effective safety testing methods. Expanding into these new markets can provide access to a broader customer base and drive demand for in vitro testing solutions. Companies that establish a presence in these regions can benefit from the growing need for advanced testing technologies and contribute to the overall growth of the market.
Trends for the In Vitro Toxicology Testing Market
Shift Toward 3D Cell Cultures
A notable trend in the in vitro toxicology testing market is the shift toward using 3D cell cultures instead of traditional 2D cultures. 3D cell cultures better mimic the complex environment of tissues and organs in the human body, providing more accurate and relevant data on how substances affect living organisms. This trend is driven by the need for more realistic testing models that can improve the predictability of safety assessments. By adopting 3D cultures, researchers can achieve better insights into the potential impacts of chemicals and drugs, leading to more reliable and effective safety evaluations.
Integration of Artificial Intelligence and Machine Learning
Artificial intelligence (AI) and machine learning are increasingly being integrated into in vitro toxicology testing. These technologies enhance the analysis of large datasets generated from tests, allowing for more precise predictions and faster results. AI algorithms can identify patterns and correlations that might be missed by traditional analysis methods, improving the accuracy of toxicity assessments. As these technologies continue to advance, they are expected to play a larger role in optimizing testing processes and interpreting results, making in vitro testing more efficient and insightful.
Focus on Human-Relevant Testing Models
There is a growing emphasis on developing human-relevant testing models in the in vitro toxicology testing market. Researchers are working to create models that closely resemble human physiology to better predict how substances will affect people. This trend includes the use of organ-on-a-chip systems and microfluidic devices that simulate human organs and tissues. These advanced models offer more accurate insights into potential human responses and help bridge the gap between laboratory tests and real-world outcomes. As the demand for more relevant testing increases, this trend is likely to shape the future of in vitro toxicology.
Increased Adoption of Alternative Testing Methods
The adoption of alternative testing methods is another significant trend. There is a growing preference for in vitro tests over traditional animal testing due to ethical concerns and regulatory pressures. Methods such as cell-based assays, biochemical tests, and high-throughput screening are gaining popularity as they offer more humane and efficient ways to evaluate safety. This trend reflects a broader movement towards reducing animal use in research and finding viable alternatives that align with ethical standards and regulatory requirements.
Emergence of Personalized Toxicology
Personalized toxicology is an emerging trend in the market, driven by the need for tailored safety assessments based on individual genetic profiles. Personalized toxicology involves testing how different people might react to substances based on their unique genetic makeup. This approach allows for more precise predictions of toxicity and safer product development. As personalized medicine continues to grow, personalized toxicology is expected to become a key component in ensuring that products are safe for diverse populations.
Growth in Emerging Markets
The in vitro toxicology testing market is also witnessing growth in emerging markets. As developing regions experience economic progress and industrial expansion, there is an increased need for advanced testing solutions to ensure product safety. These markets offer new opportunities for companies specializing in in vitro testing technologies. Expanding into these regions allows businesses to tap into new customer bases and address the rising demand for reliable and ethical safety testing methods.
Segments Covered in the Report
By Product
o Consumables
o Assays
o Instruments
o Software
o Services
o Other Product
By Technology
o Cell Culture Technology
o High Throughput Technology
o Molecular Imaging Technology
o OMICS Technology
o Other Technology
By Method
o Cellular Assay
o Live Cells
o High Throughput / High Content Screening
o Molecular Imaging
o Confocal Microscopy
o Others
o Others
o Fixed Cells
o Biochemical Assay
o In Silico
o Ex-vivo
By End-use
o Pharmaceutical Industry
o Cosmetics & Household Products
o Academic Institutes & Research Laboratories
o Diagnostics
o Medical Devices
o Others
o Chemicals Industry
o Food Industry
Segment Analysis
By Product Analysis
The consumables category dominated the market in 2023 and is expected to increase at the fastest CAGR during the forecast period due to the rising use of in-vitro toxicity testing in drug development and research. A variety of consumables are necessary for in vitro toxicology testing, including reagents, culture media, cell lines, assay plates, and other lab supplies. Many investigations require reagents, including assays used to determine the toxicity of substances or medications.

Several firms provide a diverse range of reagents and products for in vitro toxicology testing, including cell culture media and supplements, assay kits for evaluating cytotoxicity, genotoxicity, and other endpoints, and fluorescence probes and antibodies for detecting specific biomarkers. Sigma-Aldrich and Thermo Fisher Scientific are some examples of such companies.
The Assays segment is expected to grow at a significant CAGR over the forecast period. In vitro toxicity experiments aid in determining the possible effects of novel agrochemicals, food additives, medicines, and other hazardous chemical products on humans. Furthermore, strategic efforts by key market competitors are the primary driver of the segment's growth during the research period. For example, in March 2022, Toxys BV, a Dutch biotech company, established a sales office in New York and a production plant in Gaithersburg to assist its business development.
By Technology Analysis
In 2023, the cell culture technology category dominated the global market, accounting for 44.2%. Cell culture is an ideal model for researching the effects of toxins on cells because of its consistency and reproducibility. Cell culture technology aids in the early discovery of toxicity during medication development. Along with these advantages, the increasing number of investments by market participants is likely to have a beneficial impact on segment growth. For example, in March 2023, ZEISS Ventures announced an investment in InSphero to advance their research into 3D cell culture.
High throughput technology is predicted to have the fastest CAGR throughout the forecast period. The growing preference for nonmaterial safety has supplemented the demand for HTT; for example, high-throughput screening provides nonmaterial safety testing and permits the use of multiple types of cells at varying concentrations, resulting in shorter timelines, lower costs, and less fluctuation in results. Several firms provide HTT-based in vitro toxicology analysis solutions, such as Solidus Bioscience's MetaChip Technology, which uses HTT.
By Method Analysis
Cellular assay accounted for the biggest part of revenue and is predicted to expand over the projection period due to the increased availability of these techniques for pharmacokinetic profiling of medicinal products. These assays have become more widely used in recent years. Cell assays are now used in oncology research to measure both drug toxicity and tumor cell growth inhibition. In vitro toxicity cell assays have several advantages, including low cost, rapidity, and possibility for automation. Furthermore, important efforts made by manufacturers for the development of innovative cellular assays accelerate market growth.
The biochemical assay segment is expected to grow significantly over the forecast period. In vitro, biochemical assays may be used to assess acute toxicity. These assays include protein specificity, gene expression, and protein secretion. Specific protein assays determine if a therapeutic component binds to a specific receptor or biomolecule, or inhibits a specific biomolecule (enzyme), hence testing a molecular process.
By End-use Analysis
The pharmaceutical industry end-use segment dominated the market in 2023. It is the largest market for in vitro toxicology testing since it entails identifying a possible therapeutic candidate at an early stage. It is gradually operating under the three R's principle, which means that the pharmaceutical industry is prepared to replace, reduce, and refine the use of animals in drug development and toxicological profiling.
The diagnostics section is expected to grow at the quickest rate among all segments over the projection period. The presence of companies such as Toxikon, who provide tailored solutions to aid in the clinical development of diagnostic devices, contributes to the estimated share. Biocompatibility testing is performed to assess the potential side effects of medical equipment that may be hazardous to human health. Furthermore, companies have extensive product lines for detecting point-of-care drug testing, long-term alcohol misuse, and therapeutic drug monitoring, which are expected to drive segment expansion.
Regional Analysis
North America dominated the market, accounting for 48.2% in 2023. Certain factors, such as increased government attention on drug discovery, higher healthcare expenditure, and the availability of suitable infrastructure for the growth and development of drug discovery technologies, are primarily driving the North American in-vitro toxicology testing market. Furthermore, strict regulatory restrictions for medication research and licensing have raised the use of services in this geographical market.
Asia Pacific is expected to experience considerable expansion in the in vitro toxicity testing market. Countries like China, India, and South Korea are responsible for the region's total growth. China's market is expected to be the largest revenue-generating market, with India experiencing the quickest growth rate.
In vitro toxicity testing in India is becoming more competitive as pharmaceutical companies increase their testing capacity. In recent years, India has developed as a pharmaceutical research and development hub, with several international corporations establishing offices there to take advantage of its trained workforce, advanced infrastructure, and reduced costs. This tendency has resulted in a large increase in demand for in vitro toxicology testing services in India. This has resulted in the formation of several new testing companies, as well as the expansion of current market participants. For example, in January 2023, Eurofins Scientific announced the opening of a laboratory campus in Hyderabad.
Competitive Analysis
Leading competitors in the In Vitro Toxicology Testing Market are working on strategic initiatives to keep their competitive advantage. This includes product releases, collaborations, and acquisitions designed to broaden their portfolios and geographic reach. Common strategies include investing in research and market development to improve testing capabilities and satisfy growing regulatory standards. Furthermore, these players are focusing more on technical improvements, such as automation and high-throughput screening, to increase the efficiency and accuracy of toxicity testing services, hence strengthening their market position.
Recent Developments
In April 2023: Thermo Fisher Scientific released its first real-time PCR assay kit, 37 CE-IVD, for evaluating infectious illnesses.
In March 2022: WuXi AppTec increased its toxicological presence by opening a new lab in Chengdu. This expansion enabled them to provide greater service by accelerating the beginning of clinical investigations.
Key Market Players in the In Vitro Toxicology Testing Market
o Charles River Laboratories International Inc.
o SGS S.A.
o Merck KGaA
o Abbott Laboratories
o Laboratory Corporation of America Holdings
o Thermo Fisher Scientific Inc.
o Quest Diagnostics Incorporated
o Agilent Technologies Inc.
o Catalent Inc.
o Other Key Players
|
Report Features |
Description |
|
Market Size 2023 |
USD 19.1 Billion |
|
Market Size 2033 |
USD 56.0 Billion |
|
Compound Annual Growth Rate (CAGR) |
11.0% (2023-2033) |
|
Base Year |
2023 |
|
Market Forecast Period |
2024-2033 |
|
Historical Data |
2019-2022 |
|
Market Forecast Units |
Value (USD Billion) |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
By Product, Technology, Method, End-Use, and Region |
|
Geographies Covered |
North America, Europe, Asia Pacific, and the Rest of the World |
|
Countries Covered |
The U.S., Canada, Germany, France, U.K, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil |
|
Key Companies Profiled |
Charles River Laboratories International, Inc., SGS S.A., Merck KGaA, Eurofins Scientific, Abbott Laboratories, Laboratory Corporation of America Holdings, Evotec S.E., Thermo Fisher Scientific, Inc., Quest Diagnostics Incorporated, Agilent Technologies, Inc., Catalent, Inc., Other Key Players |
|
Key Market Opportunities |
Expansion of Regulatory Frameworks |
|
Key Market Dynamics |
Increased Research and Development Activities |
📘 Frequently Asked Questions
1. What would be the forecast period in the In Vitro Toxicology Testing Market?
Answer: The forecast period in the In Vitro Toxicology Testing Market report is 2024-2033.
2. How much is the In Vitro Toxicology Testing Market in 2023?
Answer: The In Vitro Toxicology Testing Market size was valued at USD 19.1 Billion in 2023.
3. Who are the key players in the In Vitro Toxicology Testing Market?
Answer: Charles River Laboratories International, Inc., SGS S.A., Merck KGaA, Eurofins Scientific, Abbott Laboratories, Laboratory Corporation of America Holdings, Evotec S.E., Thermo Fisher Scientific, Inc., Quest Diagnostics Incorporated, Agilent Technologies, Inc., Catalent, Inc., Other Key Players
4. What is the growth rate of the In Vitro Toxicology Testing Market?
Answer: In Vitro Toxicology Testing Market is growing at a CAGR of 11.0% during the forecast period, from 2023 to 2033.

🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
- Accurate & Verified Data:Our insights are trusted by global brands and Fortune 500 companies.
- Complete Transparency:No hidden fees, locked content, or misleading claims — ever.
- 24/7 Analyst Support:Our expert team is always available to help you make smarter decisions.
- Instant Savings:Enjoy a flat $1000 OFF on every report.
- Fast & Reliable Delivery:Get your report delivered within 5 working days, guaranteed.
- Tailored Insights:Customized research that fits your industry and specific goals.



