
🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
At Infinity Market Research, we dont just deliver data — we deliver clarity, confidence, and competitive edge.
In a world driven by insights, we help businesses unlock the infinite potential of informed decisions.
Here why global brands, startups, and decision-makers choose us:
Industry-Centric Expertise
With deep domain knowledge across sectors — from healthcare and technology to manufacturing and consumer goods — our team delivers insights that matter.
Custom Research, Not Cookie-Cutter Reports
Every business is unique, and so are its challenges. Thats why we tailor our research to your specific goals, offering solutions that are actionable, relevant, and reliable.
Data You Can Trust
Our research methodology is rigorous, transparent, and validated at every step. We believe in delivering not just numbers, but numbers that drive real impact.
Client-Centric Approach
Your success is our priority. From first contact to final delivery, our team is responsive, collaborative, and committed to your goals — because you re more than a client; you re a partner.
Recent Reports
Obesity Management Market
GLP-1 Receptor Agonist Market
Isotype Control Antibody Market
Global Isotype Control Antibody Market (By Disease Indication (CNS Disorders, Cardiovascular Diseases, Cancer, Auto-immune Disorders), By End-User (Hospitals, Long-term Care Facilities, Research Institutes), By Region and Companies)
Sep 2024
Healthcare
Pages: 138
ID: IMR1219
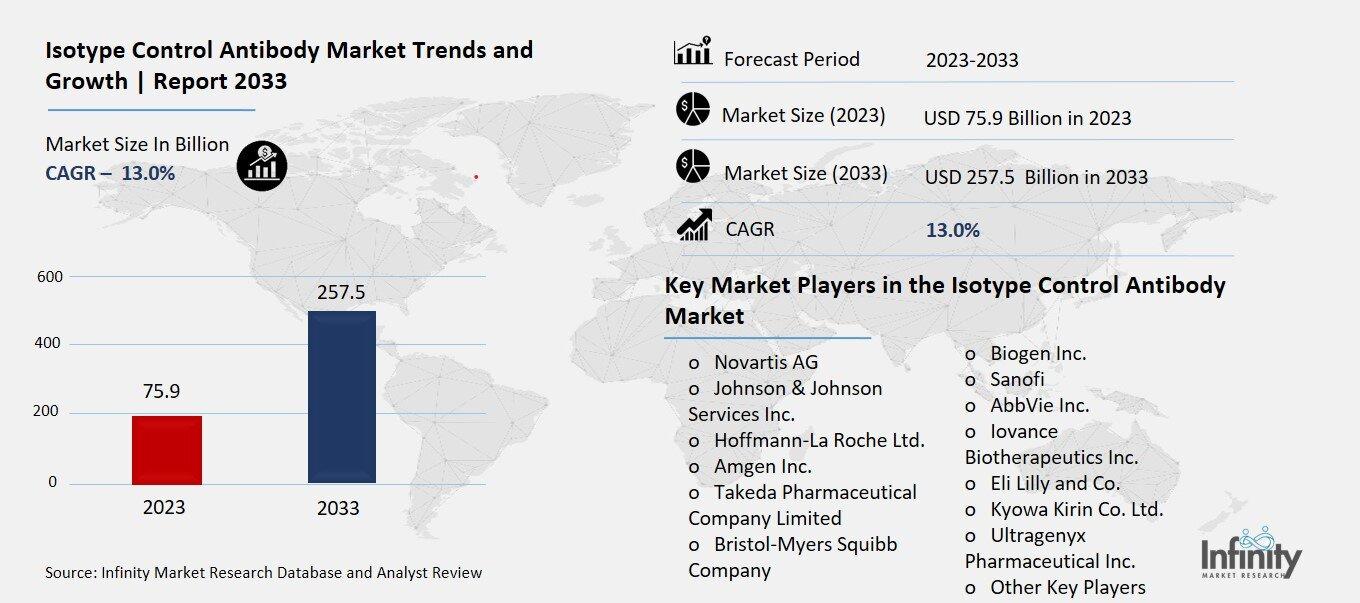
Isotype Control Antibody Market Overview
Global Isotype Control Antibody Market size is expected to be worth around USD 257.5 Billion by 2033 from USD 75.9 Billion in 2023, growing at a CAGR of 13.0% during the forecast period from 2023 to 2033.
The Isotype Control Antibody Market is about the sale and use of special antibodies in scientific research, especially in fields like medicine and biology. Isotype control antibodies are used as a "control" or baseline in experiments to help scientists make sure that the results they see are truly from the test they are running and not due to random factors or background noise. These antibodies don’t have any specific target, unlike other antibodies that might be used in the same experiment. Instead, they help researchers figure out if what they’re seeing is due to the antibodies they’re actually studying or just some accidental reactions in the experiment.
This market is important because it supports many different types of research, such as drug development, cancer research, and other medical studies. Since these antibodies help improve the reliability of scientific experiments, there is a steady demand for them in laboratories around the world. The growth of the market is driven by the expanding field of biotechnology, increasing investment in research and development, and the need for new treatments for various diseases. Overall, the Isotype Control Antibody Market plays a key role in advancing scientific discoveries by ensuring that experiments are accurate and trustworthy.
Drivers for the Isotype Control Antibody Market
Rising Demand for Immunotherapy
One of the primary drivers for the Isotype Control Antibody Market is the growing demand for immunotherapy. Immunotherapies, such as checkpoint inhibitors, CAR-T cell therapies, and therapeutic vaccines, are becoming increasingly popular due to their ability to target cancer and other chronic diseases effectively. Isotype control antibodies play a crucial role in immunotherapy by helping researchers distinguish between specific and non-specific antibody binding, which is vital for accurate analysis of immune responses. As these treatments continue to gain acceptance and become more widely available, the demand for isotype control antibodies is expected to grow.
Increasing Prevalence of Chronic Diseases
Another significant factor contributing to market growth is the increasing prevalence of chronic diseases, such as cancer, autoimmune disorders, and infectious diseases. Isotype control antibodies are essential in the development of treatments for these conditions, as they help ensure the accuracy of experiments by eliminating non-specific signals. With chronic diseases on the rise globally, especially in aging populations, there is a growing need for more precise and targeted therapies, which in turn drives the demand for isotype control antibodies.
Advancements in Antibody Engineering
Advancements in antibody engineering technologies have also played a crucial role in boosting the market. New techniques in bioinformatics and high-throughput screening are allowing for the creation of isotype control antibodies with enhanced specificity, functionality, and performance. These advancements help reduce cross-reactivity, improve diagnostic accuracy, and enhance therapeutic efficacy, thereby increasing the adoption of these antibodies in research and clinical applications.
Growing Investment in Biomedical Research
There is an increasing investment in biomedical research globally, especially in regions such as North America, Europe, and Asia-Pacific. This trend is largely driven by the need for innovative solutions to manage and treat complex diseases. Isotype control antibodies are a key tool in this research, used to develop new diagnostic tests and therapeutic approaches. Governments, academic institutions, and private entities are pouring substantial funds into research and development (R&D), further propelling the market for isotype control antibodies.
Expansion of the Biotechnology and Life Sciences Sector
The expansion of the biotechnology and life sciences sectors in emerging markets, particularly in Asia-Pacific, is another driver. Countries like China, India, and South Korea are investing heavily in infrastructure and research, making them attractive locations for clinical trials and drug development activities. The growing biotech industry in these regions is fostering demand for isotype control antibodies for use in various research applications, from drug discovery to clinical testing.
Increasing Partnerships and Collaborations
Finally, strategic partnerships and collaborations between pharmaceutical companies, research institutes, and universities are promoting the development and utilization of isotype control antibodies. Such collaborations enable the pooling of resources and expertise, accelerating the pace of research and increasing the use of isotype control antibodies in both preclinical and clinical trials.
Restraints for the Isotype Control Antibody Market
High Cost of Antibodies
One of the significant restraints in the isotype control antibody market is the high cost associated with the development and production of these antibodies. Isotype control antibodies, which are essential in research and diagnostic procedures, are expensive due to the complex processes involved in their creation. These antibodies require specialized equipment, raw materials, and skilled professionals, which all add to the cost. As a result, smaller research institutions and healthcare facilities may find it challenging to afford these products, limiting the market's growth potential.
Stringent Regulatory Frameworks
The isotype control antibody market is also restrained by stringent regulatory frameworks. These antibodies are classified as biological products, which means they must comply with strict guidelines set by regulatory authorities like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These regulations are in place to ensure safety and efficacy, but they also add time and cost to the product development cycle. Companies often face long approval processes, which can delay the market entry of new products and increase the overall cost of development.
Competition from Alternative Technologies
Competition from alternative technologies is another restraint for the isotype control antibody market. New techniques and alternatives such as synthetic antibodies, nanobodies, and aptamers are gaining popularity due to their cost-effectiveness and ease of production. These alternatives provide similar or better functionality, which reduces the demand for traditional isotype control antibodies. As research laboratories and healthcare institutions look for more affordable options, the market for isotype control antibodies faces growing competition, potentially impacting market growth.
Limited Awareness and Adoption
Another challenge in the market is limited awareness and adoption, particularly in emerging regions. In many countries, there is a lack of knowledge regarding the importance and applications of isotype control antibodies in research and diagnostic procedures. This limited awareness results in lower adoption rates and reduced demand, particularly in regions where research activities are less developed or poorly funded. As a result, market growth may be constrained due to a lack of awareness and understanding among potential users.
Supply Chain Disruptions
Supply chain disruptions also act as a restraint in the isotype control antibody market. The market relies heavily on a continuous supply of raw materials, and any disruption such as geopolitical tensions, trade restrictions, or pandemics can impact the production and supply of these antibodies. Delays or shortages in raw materials can increase production costs and affect the timely delivery of products to customers, thereby restraining market growth.
Opportunity in the Isotype Control Antibody Market
Rising Demand from Research and Diagnostics
One of the main opportunities in the Isotype Control Antibody Market comes from the increasing demand for research and diagnostic applications. Isotype control antibodies are critical in experiments involving flow cytometry and immunohistochemistry to ensure specificity and accuracy. As these techniques become more common in both academic and commercial laboratories, the demand for high-quality isotype control antibodies is expected to grow.
Growth in Healthcare Facilities
Another significant opportunity lies in the expanding use of isotype control antibodies across diverse healthcare settings, including hospitals and long-term care facilities. With the growing prevalence of chronic diseases like cancer, cardiovascular diseases, and auto-immune disorders, there is an increasing need for accurate diagnostic tools. This demand is particularly strong in regions with advanced healthcare infrastructures, such as North America and Europe.
Development of Personalized Medicine
The evolution of personalized medicine offers a substantial opportunity for growth in the isotype control antibody market. Personalized medicine, which tailors medical treatment to the individual characteristics of each patient, relies heavily on precision diagnostics and therapeutic approaches. Isotype control antibodies play a critical role in ensuring the accuracy of these diagnostic tests, driving their demand even further.
Expansion in Emerging Markets
Emerging markets in Asia-Pacific, Latin America, and the Middle East & Africa present a significant growth opportunity due to increasing healthcare spending, improving healthcare infrastructure, and growing awareness of advanced diagnostic techniques. As these regions continue to develop, the market for isotype control antibodies is expected to expand, providing opportunities for companies to establish a strong foothold.
Strategic Collaborations and Partnerships
Collaborations and partnerships among key players in the isotype control antibody market, such as Novartis AG, Hoffmann-La Roche Ltd., Johnson & Johnson Services, Inc., and others, are creating opportunities for innovation and growth. These strategic alliances facilitate the development of new products and enhance market reach, enabling companies to address diverse customer needs across the globe.
Technological Advancements
Finally, technological advancements in antibody production and development are creating new opportunities in the market. Innovations such as recombinant antibody technology and the development of novel isotype control antibodies with enhanced specificity and reduced non-specific binding are likely to drive market growth in the coming years.
Trends for the Isotype Control Antibody Market
Rising Demand in Immunotherapy Research
Immunotherapy, including treatments like CAR-T cell therapy and therapeutic vaccines, is a major driver for the use of isotype control antibodies. These antibodies help researchers accurately measure immune responses, which is crucial in developing new cancer therapies and treatments for autoimmune diseases. The increasing need for precision in these areas propels the demand for high-quality isotype control antibodies to distinguish specific immune reactions from background noise.
Expanding Role in Biomarker Discovery
Isotype control antibodies are also becoming essential in biomarker discovery and validation, which are key components of personalized medicine. Biomarkers help identify which patients will respond best to specific treatments, making the use of isotype control antibodies in research and clinical settings highly valuable. The growing focus on personalized medicine and the need for specific diagnostic tools are driving this trend forward.
Technological Advancements in Antibody Production
Technological advancements, such as recombinant antibody production and high-throughput screening methods, have significantly enhanced the quality and specificity of isotype control antibodies. These innovations are reducing the time and cost associated with developing new antibodies, which encourages more extensive use across various research fields, including oncology, immunology, and infectious diseases.
Increasing Adoption in Clinical and Preclinical Research
The use of isotype control antibodies in clinical and preclinical research is expanding. They are used extensively in experiments to validate the effectiveness and safety of new therapeutic agents. This trend is particularly notable in cancer research, where isotype control antibodies help evaluate the immune response to tumors, leading to more effective treatments and therapies. As a result, pharmaceutical companies and research institutions are investing heavily in these antibodies to support ongoing studies and trials.
Growing Market Opportunities in Asia-Pacific
The Asia-Pacific region is expected to see the fastest growth in the Isotype Control Antibody market due to several factors, such as a large patient population, diverse genetic backgrounds, and lower operational costs for clinical trials. Countries like China, India, and Japan are investing in antibody engineering and biotechnological research, fostering market expansion in this region. This trend is further supported by collaborations between regional research institutes and global pharmaceutical companies.
Strengthening Collaborations and Partnerships
Collaborations between biotechnology firms, academic institutions, and pharmaceutical companies are becoming more common, leading to innovative developments in antibody research. These partnerships often focus on co-developing new antibodies, optimizing production processes, and enhancing the clinical applicability of isotype control antibodies. As these collaborations continue to grow, they contribute significantly to the overall expansion of the market, driving further research and innovation.
Segments Covered in the Report
By Disease Indication
o CNS Disorders
o Cardiovascular Diseases
o Cancer
o Auto-immune Disorders
By End-User
o Hospitals
o Long-term Care Facilities
o Research Institutes
Segment Analysis
By Disease Indication Analysis
The Isotype Control Antibody Market is segmented by illness indication, including CNS disorders, cardiovascular diseases, cancer, and auto-immune disorders. In 2023, the cancer sector dominated the market. Isotype control antibodies are critical in the development of cancer immunotherapies such as CAR-T cell treatments, immune system checkpoint medicines, and therapeutic vaccines. They serve as crucial negative controls in preclinical and clinical studies to determine therapy efficacy and safety. Isotype control antibodies are used in studies to identify potential biomarkers associated with cancer diagnosis, prognosis, and therapy response. They allow researchers to assess the clinical utility of potential biomarker candidates for patient classification and personalized treatment.
The cardiovascular disease category is expected to be the fastest increasing. Immunohistochemistry (IHC) and immunofluorescence (IF) procedures use isotype control antibodies to locate and detect proteins associated with cardiovascular disease. They contribute to the appropriate interpretation of experimental results by serving as negative controls for antibody specificity and non-specific background staining in tissue samples.
By End-User Analysis
The Isotype Control Antibody Market is segmented by End-User, including hospitals, long-term care institutions, and research institutes. In 2023, the hospital category made the highest revenue. Isotype control antibodies are used to monitor disease progression and predict the prognosis of patients with a variety of medical conditions, including infectious diseases, auto-immune disorders, and cancer. Hospitals utilize these antibodies in tests to analyze immune cell populations and biomarker expression levels, which provides valuable information for medication selection. Through research and development programs, hospitals strive to enhance patient care and increase medical knowledge. Isotype control antibodies are critical tools in hospital-based research laboratories for determining disease origins, evaluating therapy options, and developing novel therapeutic and diagnostic tests.
The Isotype Control Antibody Research Institutes sector market is expected to expand the fastest during the projected period. Cancer research facilities extensively use isotype control antibodies to study immune responses, tumor biology, and therapies. Researchers utilize these antibodies in preclinical models and clinical trials to validate experimental results, assess treatment efficacy, and follow disease progression. This improves cancer diagnosis and treatment.
Regional Analysis
The North American Isotype Control Antibody market will lead this market. North America, particularly the United States, is home to a thriving biopharmaceutical industry characterized by extensive, creative work exercises. The district's liberal biotechnology environment, along with a strong interest in biomedical research, encourages interest in isotype control antibodies as critical assets for the discovery and development of drugs. North America boasts several highly regarded research associations, schools, and academic clinical offices dedicated to cutting-edge biomedical research. These companies generate revenue from isotype control antibodies in a variety of examination fields, including immunology, cancer, infectious diseases, and neuroscience.
Europe has the second-largest market share for isotype control antibodies. European countries play an important role in sponsoring and supporting biomedical research through a variety of programs and funding bodies. This helps increase the demand for isotype control antibodies in both academic and commercial research projects. Furthermore, the German Isotype Control Antibody market had the highest market share, while the UK Isotype Control Antibody market was the fastest-growing in the European region.
The Asia-Pacific Isotype Control Antibody Market is estimated to expand at the quickest CAGR between 2023 and 2033. The Asia-Pacific region is experiencing an increase in the prevalence of chronic diseases such as cancer, diabetes, and cardiovascular infections. This has sparked a growing interest in isotype control antibodies for disease research, biomarker discovery, and drug development. Furthermore, China's Isotype Control Antibody market had the highest market share, while India's Isotype Control Antibody market was the fastest-growing in the Asia-Pacific region.
Competitive Analysis
The Isotype Control Antibody market is highly competitive, with several established players and new entrants striving to gain a foothold. Major companies like Thermo Fisher Scientific, BD Biosciences, Bio-Rad Laboratories, and Abcam dominate the market due to their extensive product portfolios, advanced technology, and strong distribution networks. These companies invest heavily in research and development to enhance the quality and specificity of their antibodies, catering to the growing demand from the pharmaceutical and biotechnology sectors. Their focus on innovation, coupled with strategic acquisitions and partnerships, allows them to maintain a competitive edge and expand their market presence globally.
Key Market Players in the Isotype Control Antibody Market
o Novartis AG
o Johnson & Johnson Services Inc.
o Hoffmann-La Roche Ltd.
o Amgen Inc.
o Takeda Pharmaceutical Company Limited
o Bristol-Myers Squibb Company
o Biogen Inc.
o Sanofi
o AbbVie Inc.
o Iovance Biotherapeutics Inc.
o Eli Lilly and Co.
o Kyowa Kirin Co. Ltd.
o Ultragenyx Pharmaceutical Inc.
o Other Key Players
|
Report Features |
Description |
|
Market Size 2023 |
USD 75.9 Billion |
|
Market Size 2033 |
USD 257.5 Billion |
|
Compound Annual Growth Rate (CAGR) |
13.0% (2023-2033) |
|
Base Year |
2023 |
|
Market Forecast Period |
2024-2033 |
|
Historical Data |
2019-2022 |
|
Market Forecast Units |
Value (USD Billion) |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
By Disease Indication, End-User, and Region |
|
Geographies Covered |
North America, Europe, Asia Pacific, and the Rest of the World |
|
Countries Covered |
The U.S., Canada, Germany, France, U.K, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil |
|
Key Companies Profiled |
Novartis AG, Johnson & Johnson Services Inc., Hoffmann-La Roche Ltd., Amgen Inc., Takeda Pharmaceutical Company Limited, Bristol-Myers Squibb Company, Biogen Inc., Sanofi, AbbVie Inc., Iovance Biotherapeutics Inc., Eli Lilly and Co., Kyowa Kirin Co. Ltd., Ultragenyx Pharmaceutical Inc., Other Key Players |
|
Key Market Opportunities |
Rising Demand from Research and Diagnostics |
|
Key Market Dynamics |
Rising Demand for Immunotherapy |
📘 Frequently Asked Questions
1. Who are the key players in the Isotype Control Antibody Market?
Answer: Novartis AG, Johnson & Johnson Services Inc., Hoffmann-La Roche Ltd., Amgen Inc., Takeda Pharmaceutical Company Limited, Bristol-Myers Squibb Company, Biogen Inc., Sanofi, AbbVie Inc., Iovance Biotherapeutics Inc., Eli Lilly and Co., Kyowa Kirin Co. Ltd., Ultragenyx Pharmaceutical Inc., Other Key Players
2. How much is the Isotype Control Antibody Market in 2023?
Answer: The Isotype Control Antibody Market size was valued at USD 75.9 Billion in 2023.
3. What would be the forecast period in the Isotype Control Antibody Market?
Answer: The forecast period in the Isotype Control Antibody Market report is 2023-2033.
4. What is the growth rate of the Isotype Control Antibody Market?
Answer: Isotype Control Antibody Market is growing at a CAGR of 1.0% during the forecast period, from 2023 to 2033.

🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
- Accurate & Verified Data:Our insights are trusted by global brands and Fortune 500 companies.
- Complete Transparency:No hidden fees, locked content, or misleading claims — ever.
- 24/7 Analyst Support:Our expert team is always available to help you make smarter decisions.
- Instant Savings:Enjoy a flat $1000 OFF on every report.
- Fast & Reliable Delivery:Get your report delivered within 5 working days, guaranteed.
- Tailored Insights:Customized research that fits your industry and specific goals.



