
🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
At Infinity Market Research, we dont just deliver data — we deliver clarity, confidence, and competitive edge.
In a world driven by insights, we help businesses unlock the infinite potential of informed decisions.
Here why global brands, startups, and decision-makers choose us:
Industry-Centric Expertise
With deep domain knowledge across sectors — from healthcare and technology to manufacturing and consumer goods — our team delivers insights that matter.
Custom Research, Not Cookie-Cutter Reports
Every business is unique, and so are its challenges. Thats why we tailor our research to your specific goals, offering solutions that are actionable, relevant, and reliable.
Data You Can Trust
Our research methodology is rigorous, transparent, and validated at every step. We believe in delivering not just numbers, but numbers that drive real impact.
Client-Centric Approach
Your success is our priority. From first contact to final delivery, our team is responsive, collaborative, and committed to your goals — because you re more than a client; you re a partner.
Recent Reports
Obesity Management Market
GLP-1 Receptor Agonist Market
Oral Thin Film Drugs Market
Oral Thin Film Drugs Market (By Product (Fast Dissolving Buccal Film and Sublingual ), By Disease Indication (Opioid Dependence, Nausea & Vomiting, Schizophrenia, Migraine), By Distribution Channel (Retail Pharmacies, Hospital Pharmacies, Online Drug Stores), By Region and Companies)
Jun 2024
Healthcare
Pages: 103
ID: IMR1097
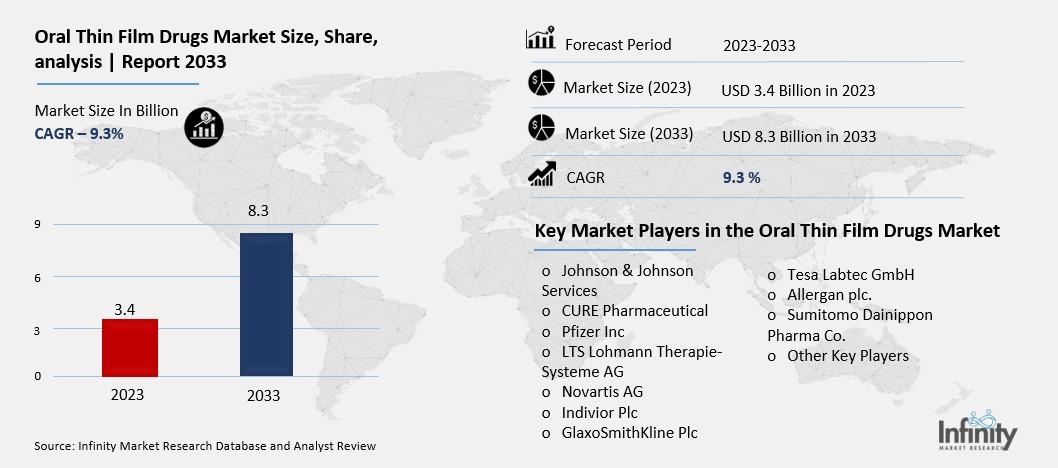
Oral Thin Film Drugs Market Overview
Global Oral Thin Film Drugs Market size is expected to be worth around USD 8.3 Billion by 2033 from USD 3.4 Billion in 2023, growing at a CAGR of 9.3% during the forecast period from 2023 to 2033.
Oral thin film drugs are a type of medication delivery system where the drug is embedded in a thin film that dissolves when placed in the mouth. This market is growing because these films offer a convenient and effective way to administer drugs, especially for people who have trouble swallowing pills. The global market is driven by the rising demand for innovative drug delivery systems, the increasing prevalence of diseases that benefit from this delivery method, and advancements in technology that make production more efficient and effective. Companies are investing in research and development to create new products and improve existing ones, aiming to capture a larger share of this expanding market
Drivers for the Oral Thin Film Drugs Market
Patient Convenience and Preference:
One of the primary drivers for the oral thin film drugs market is patient convenience. Many patients prefer oral thin films over traditional tablets or injections due to ease of administration. These films dissolve quickly in the mouth, making them suitable for patients who have difficulty swallowing pills or need medication when access to water is limited. This convenience factor is particularly significant in pediatric and geriatric populations, where compliance with medication regimens can be challenging.
Technological Advancements in Drug Delivery:
Advancements in pharmaceutical formulation technologies have significantly contributed to the growth of the oral thin film drugs market. Manufacturers are investing in research and development to enhance the bioavailability, stability, and taste-masking capabilities of oral thin films. This has led to the development of films that can deliver a wide range of drugs, including over-the-counter medications, prescription drugs, and even vaccines.
Expanding Therapeutic Applications:
The market for oral thin film drugs is expanding into various therapeutic areas, including pain management, central nervous system disorders, and hormonal therapies. The versatility of oral thin films allows for the delivery of both small-molecule drugs and biologics, opening up new opportunities for pharmaceutical companies to address unmet medical needs.
Regulatory Support and Market Access:
Regulatory agencies, such as the FDA in the United States and the EMA in Europe, have been supportive of the development and commercialization of oral thin film drugs. This support has streamlined the approval process for new products, enabling faster market access for manufacturers. Additionally, the global market for oral thin films is benefiting from increasing healthcare expenditure and the growing prevalence of chronic diseases, which are driving demand for innovative drug delivery solutions.
Market Growth and Investment Opportunities:
The oral thin film drugs market is witnessing significant growth, with several pharmaceutical companies expanding their product portfolios to include oral thin films. Investment in manufacturing facilities and partnerships with contract development and manufacturing organizations (CDMOs) are also contributing to market growth. As a result, the oral thin film drugs market is projected to continue expanding, offering new investment opportunities in the pharmaceutical sector.
Restraints for the Oral Thin Film Drugs Market
Manufacturing Complexities and Cost Considerations:
One of the primary restraints for the oral thin film drugs market is the complexity and cost associated with manufacturing these films. Developing oral thin films requires specialized equipment and expertise, which can drive up production costs. Additionally, ensuring uniformity in dose delivery and maintaining the stability of active pharmaceutical ingredients (APIs) in the film matrix adds to manufacturing complexities. These factors can make oral thin film drugs more expensive to produce compared to traditional dosage forms.
Regulatory Challenges and Approval Processes:
Regulatory approval is another significant restraint for the oral thin film drugs market. While regulatory agencies like the FDA and EMA support the development of innovative drug delivery systems, obtaining approvals for new formulations can be challenging. Companies must demonstrate the safety, efficacy, and quality of oral thin films through extensive clinical trials and regulatory submissions, which can be time-consuming and costly. Regulatory requirements vary across different regions, further complicating the approval process and delaying market entry.
Competition from Established Drug Delivery Methods:
The oral thin film drugs market faces stiff competition from other well-established drug delivery methods, such as tablets, capsules, and injections. These traditional methods have a long history of use and proven efficacy, making it challenging for oral thin films to gain market share. Physicians and patients may be more accustomed to these conventional dosage forms, which can affect the adoption of oral thin film drugs, particularly in therapeutic areas where alternatives are widely available.
Limitations in Drug Compatibility and Formulation:
Another restraint for the oral thin film drugs market is the limited compatibility of certain drugs with the film formulation. Oral thin films must be designed to accommodate specific APIs, and not all drugs are suitable for this delivery method. Challenges such as poor solubility, stability issues, and taste masking can limit the types of drugs that can be effectively delivered via oral thin films. This restricts the market potential for these films in certain therapeutic areas and limits their applicability to a narrower range of medications.
Market Access and Distribution Challenges:
Market access and distribution pose additional challenges for the oral thin film drugs market. Establishing a robust supply chain and ensuring widespread availability of these products can be difficult, especially in regions with limited infrastructure or regulatory barriers. Distribution networks for oral thin films may not be as well-developed as those for traditional dosage forms, which can affect product accessibility and uptake.
Opportunity in the Oral Thin Film Drugs Market
Expanding Applications and Therapeutic Areas:
One of the key opportunities for the oral thin film drugs market lies in its expanding applications across various therapeutic areas. These films can deliver a wide range of medications, including pain management, neurological disorders, hormonal therapies, and cardiovascular diseases. As pharmaceutical companies continue to innovate and improve film formulations, the market is expected to witness increased adoption in both prescription and over-the-counter segments. This expansion is fueled by the versatility of oral thin films in delivering different types of drugs, including small molecules and biologics, providing a promising opportunity to address unmet medical needs.
Patient Preferences and Convenience:
Another significant opportunity for the oral thin film drugs market is the growing preference for non-invasive and patient-friendly drug delivery methods. Oral thin films dissolve quickly in the mouth without the need for water, making them convenient for patients who have difficulty swallowing pills or injections. This ease of administration is particularly advantageous in pediatric and geriatric populations, where compliance with medication regimens can be challenging. As patient awareness and acceptance of these innovative dosage forms increase, the market opportunity for oral thin films is expected to expand further.
Technological Advancements and Innovation:
Advancements in pharmaceutical formulation technologies present a substantial opportunity for the oral thin film drugs market. Manufacturers are investing in research and development to enhance the bioavailability, stability, and taste-masking capabilities of these films. This ongoing innovation is driving the development of novel drug combinations, controlled-release formulations, and improved therapeutic efficacy. As technology continues to evolve, oral thin films are expected to become increasingly attractive for pharmaceutical companies looking to differentiate their products and capture market share.
Global Healthcare Expenditure and Market Growth:
The global increase in healthcare expenditure is also creating favorable conditions for the oral thin film drugs market. As healthcare systems worldwide face the challenges of an aging population and rising chronic diseases, there is a growing demand for cost-effective and efficient drug delivery solutions. Oral thin films offer potential cost savings by improving medication adherence and reducing hospital admissions related to non-compliance. This economic advantage positions oral thin films as a valuable opportunity in the pharmaceutical market, especially in regions with increasing healthcare investments.
Regulatory Support and Market Access:
Regulatory agencies, such as the FDA and EMA, are supportive of innovative drug delivery systems like oral thin films, which streamline the approval process for new products. This regulatory support enhances market access and encourages pharmaceutical companies to invest in developing oral thin film drugs. Moreover, partnerships with contract development and manufacturing organizations (CDMOs) are facilitating market entry by providing expertise in formulation development, manufacturing, and regulatory compliance.
Trends for the Oral Thin Film Drugs Market
Technological Advancements in Drug Formulation:
One of the prominent trends in the oral thin film drugs market is the continuous advancement in drug formulation technologies. Manufacturers are focusing on improving the bioavailability, stability, and drug release profiles of oral thin films. Innovations in formulation techniques, such as nanotechnology and microencapsulation, are enabling the development of films that can deliver a broader range of drugs, including poorly soluble compounds and biologics. These advancements are enhancing the therapeutic efficacy and patient compliance of oral thin film drugs, contributing to their growing acceptance in the pharmaceutical industry.
Increasing Demand for Patient-Centric Drug Delivery Systems:
There is a growing trend towards patient-centric drug delivery systems, which is driving the adoption of oral thin films. Patients prefer non-invasive and easy-to-administer dosage forms, such as oral thin films, over conventional tablets or injections. These films dissolve quickly in the mouth, making them suitable for patients who have difficulty swallowing pills or need medication when access to water is limited. As patient awareness and acceptance of these innovative drug delivery methods increase, the market demand for oral thin films is expected to rise, especially in pediatric and geriatric populations.
Expanding Applications Across Therapeutic Areas:
Another significant trend in the oral thin film drugs market is the expanding applications across various therapeutic areas. Initially used for over-the-counter medications and oral care products, oral thin films are now being developed for prescription drugs in therapeutic areas such as pain management, central nervous system disorders, hormonal therapies, and cardiovascular diseases. This diversification in applications is driven by the versatility of oral thin films in delivering different types of drugs, offering pharmaceutical companies new opportunities to address unmet medical needs and expand their product portfolios.
Focus on Taste-Masking and Patient Experience:
Taste-masking and improving the patient experience are emerging trends in the development of oral thin films. Manufacturers are investing in technologies to enhance the taste and mouthfeel of these films, making them more palatable and pleasant for patients. Techniques such as flavoring agents, sweeteners, and thin film coating technologies are being utilized to mask the bitter taste of active pharmaceutical ingredients (APIs) and improve patient compliance. This focus on enhancing the sensory attributes of oral thin films is expected to further drive their adoption and market growth.
Regulatory Support and Market Expansion:
Regulatory agencies are increasingly supportive of innovative drug delivery systems like oral thin films, which are facilitating market expansion. The FDA and EMA have streamlined the approval processes for new oral thin film products, encouraging pharmaceutical companies to invest in their development. This regulatory support, coupled with partnerships with contract development and manufacturing organizations (CDMOs), is enabling faster market entry and scalability of oral thin film drugs. As a result, the market is witnessing a growing number of product launches and collaborations aimed at capitalizing on the expanding opportunities in the pharmaceutical sector.
Segments Covered in the Report
By Product
o Fast Dissolving Buccal Film
o Sublingual
By Disease Indication
o Opioid Dependence
o Nausea & Vomiting
o Schizophrenia
o Migraine
By Distribution Channel
o Retail Pharmacies
o Hospital Pharmacies
o Online Drug Stores
Segment Analysis
By Product Analysis
Oral thin-film drug products are categorized into two segments: sublingual and fast-dissolving buccal film. Owing to increasing demand and adoption of oral medication, the sublingual category retained the most significant share in 2023, accounting for over 66.2% of the oral thin film drugs market revenue. Sublingual films are tiny, sticky sheets used to absorb medication under the tongue. They can avoid the necessity for swallowing by delivering medications and vaccinations straight into the bloodstream. The sublingual film, often known as a transdermal patch or gum, is a type of drug administration that enters the skin to administer medication and allows target organs to absorb it quickly. It is possible to place sublingual films under the tongue for systemic disintegration or absorption. The increasing use of this segment in emergency treatment and its rapid adoption are the main drivers of its growth.
By Disease Indication Analysis
The oral thin-film drugs market is divided into four segments based on disease indication: migraine, nauseous vomiting, schizophrenia, and opioid dependency. In 2023, the schizophrenia segment held a dominant position in the market, and it is anticipated to develop at a faster rate over the forecast period of 2023-2033. Owing to underlying characteristics such as difficulty swallowing liquids and tablets, rapid medication absorption into the mucosal membrane, and ease of administration, drug delivery is commonly employed in patients with schizophrenia. Patients with migraines also favor this medicine delivery strategy because of its efficiency and quick alleviation of pain.
By Distribution Channel Analysis
The oral thin-film drugs market data has been divided into three segments by distribution channels: hospital pharmacies, retail pharmacies, and internet pharmacies. Due to end-user preferences, easy access to a wide variety of products, and an increase in the number of retail pharmacies in developing countries, retail pharmacies dominated the market in 2023 and are predicted to grow at the fastest rate during the projected period of 2023–2033. This segment's dominance was partly owing to pharmaceutical products' increased accessibility through retail channels.
Regional Analysis
The oral thin-film drug market in North America is anticipated to rise at a notable CAGR over the forecast period, with the region holding the largest share of 45.58% in 2023. In comparison to other regional areas, the region's market is primarily driven by the availability of products, a significant number of suppliers, and a larger penetration of oral thin-film medications. Furthermore, it is anticipated that in the upcoming years, the growing incidence of schizophrenia in industrialized nations like the US will support regional market expansion.
The second-largest market share is held by the oral thin-film drugs market in Europe. The growing prevalence of Parkinson's disease is one of many factors influencing the market's growth in Europe. Furthermore, it is anticipated that increased research and development efforts on thin-film medications will fuel market expansion in European nations. Furthermore, the oral thin-film drugs market is anticipated to be driven in the upcoming years by increased European government investment in novel drug delivery technologies. In addition, the oral thin-film drugs market in Germany had the most market share, while the oral thin-film drugs market in the UK was expanding at the quickest rate in the European Union.
From 2023 to 2033, the Asia-Pacific Oral Thin Film Drugs Market is anticipated to expand at the fastest rate. Increased healthcare spending, a greater number of older patients, and better infrastructure all contribute to this region's market expansion. Throughout the projected period, a rise in awareness of thin-film therapeutic applications is anticipated to drive the market. The regional growth of the oral thin-film medication sector is also being influenced by the introduction of new products, a greater concentration of producers or market participants, partnerships, acquisitions, and an increase in the number of target disease cases in the area. Furthermore, the oral thin-film drugs market in China accounted for the highest proportion of the Asia-Pacific market, while the oral thin-film drug market in India grew at the fastest rate.
Competitive Analysis
To strengthen their competitive edge through the introduction of innovative platform technologies, such as soluleaves, XGEL, and waferTab, major market participants in the oral thin film drug business are investing in the research and development of fast-dissolving oral thin film pharmaceuticals. New medication delivery strategies that are more practical, advantageous, and effective are developed with the help of research and development. To expand their customer base, prominent market players employ many essential methods such as contractual agreements, product releases that are unique, mergers and acquisitions, and a solid pipeline of compounds.
Key Market Players in the Oral Thin Film Drugs Market
o Johnson & Johnson Services
o Pfizer Inc
o LTS Lohmann Therapie-Systeme AG
o Indivior Plc
o GlaxoSmithKline Plc
o Allergan plc.
o Sumitomo Dainippon Pharma Co.
o Other Key Players
|
Report Features |
Description |
|
Market Size 2023 |
USD 3.4 Billion |
|
Market Size 2033 |
USD 8.3 Billion |
|
Compound Annual Growth Rate (CAGR) |
9.3% (2023-2033) |
|
Base Year |
2023 |
|
Market Forecast Period |
2024-2033 |
|
Historical Data |
- |
|
Market Forecast Units |
Value (USD Billion) |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
Product, Disease Indication, Distribution Channel, and Region |
|
Geographies Covered |
North America, Europe, Asia Pacific, and the Rest of the World |
|
Countries Covered |
The U.S., Canada, Germany, France, U.K, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil |
|
Key Companies Profiled |
Johnson & Johnson Services, CURE Pharmaceutical, Pfizer Inc, LTS Lohmann Therapie-Systeme AG, Novartis AG, Indivior Plc, GlaxoSmithKline Plc, Tesa Labtec GmbH, Allergan plc., Sumitomo Dainippon Pharma Co., Other Key Players |
|
Key Market Opportunities |
Expanding Applications and Therapeutic Areas |
|
Key Market Dynamics |
Patient Convenience and Preference |
📘 Frequently Asked Questions
1. How much is the Oral Thin Film Drugs Market in 2023?
Answer: The Oral Thin Film Drugs Market size was valued at USD 3.4 Billion in 2023.
2. What would be the forecast period in the Oral Thin Film Drugs Market report?
Answer: The forecast period in the Oral Thin Film Drugs Market report is 2023-2033.
3. Who are the key players in the Oral Thin Film Drugs Market?
Answer: Johnson & Johnson Services, CURE Pharmaceutical, Pfizer Inc, LTS Lohmann Therapie-Systeme AG, Novartis AG, Indivior Plc, GlaxoSmithKline Plc, Tesa Labtec GmbH, Allergan plc., Sumitomo Dainippon Pharma Co., Other Key Players
4. What is the growth rate of the Oral Thin Film Drugs Market?
Answer: Oral Thin Film Drugs Market is growing at a CAGR of 9.3% during the forecast period, from 2023 to 2033.

🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
- Accurate & Verified Data:Our insights are trusted by global brands and Fortune 500 companies.
- Complete Transparency:No hidden fees, locked content, or misleading claims — ever.
- 24/7 Analyst Support:Our expert team is always available to help you make smarter decisions.
- Instant Savings:Enjoy a flat $1000 OFF on every report.
- Fast & Reliable Delivery:Get your report delivered within 5 working days, guaranteed.
- Tailored Insights:Customized research that fits your industry and specific goals.



