
🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
At Infinity Market Research, we dont just deliver data — we deliver clarity, confidence, and competitive edge.
In a world driven by insights, we help businesses unlock the infinite potential of informed decisions.
Here why global brands, startups, and decision-makers choose us:
Industry-Centric Expertise
With deep domain knowledge across sectors — from healthcare and technology to manufacturing and consumer goods — our team delivers insights that matter.
Custom Research, Not Cookie-Cutter Reports
Every business is unique, and so are its challenges. Thats why we tailor our research to your specific goals, offering solutions that are actionable, relevant, and reliable.
Data You Can Trust
Our research methodology is rigorous, transparent, and validated at every step. We believe in delivering not just numbers, but numbers that drive real impact.
Client-Centric Approach
Your success is our priority. From first contact to final delivery, our team is responsive, collaborative, and committed to your goals — because you re more than a client; you re a partner.
Recent Reports
Obesity Management Market
GLP-1 Receptor Agonist Market
Respiratory Pathogen Testing Kits Market
Respiratory Pathogen Testing Kits Market (By Product (RT PCR Kits, DFA Kits, ELISA Kits, Others Source), By Technology (, NAAT, Immunoassays, Others Technology), By Infection/Symptom (Matrix, Enterovirus Infection, Human Coronavirus Infection, Influenza Virus Infection, Respiratory Syncytial Virus Infection, Rhinovirus Infection, Pneumonia), By End-User (Hospitals, Diagnostic Centers, Specialty Clinics, Other End-User), By Region and Companies)
Aug 2024
Healthcare
Pages:
ID: IMR1202
Respiratory Pathogen Testing Kits Market Overview
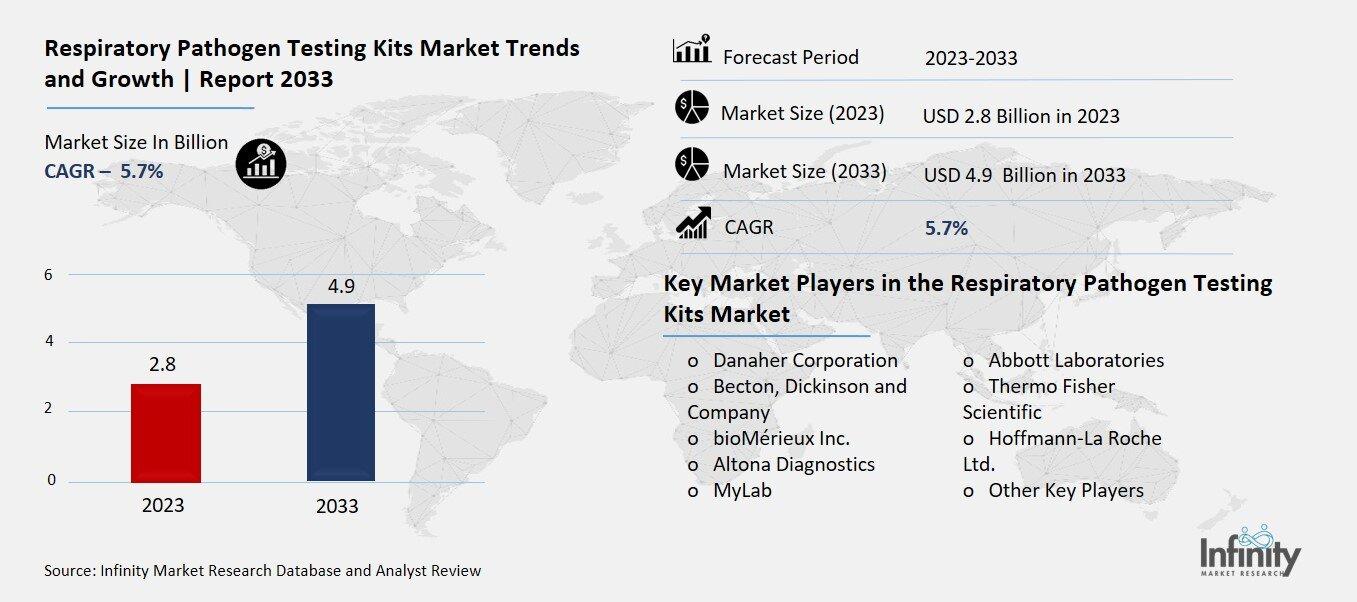
Global Respiratory Pathogen Testing Kits Market size is expected to be worth around USD 4.9 Billion by 2033 from USD 2.8 Billion in 2023, growing at a CAGR of 5.7% during the forecast period from 2023 to 2033.
The Respiratory Pathogen Testing Kits Market is all about the tools and kits used by doctors and healthcare workers to find out if someone has an infection in their lungs or breathing system. These kits help identify viruses, bacteria, or other germs that can cause illnesses like the flu, pneumonia, COVID-19, or other respiratory diseases. The market includes the companies that make and sell these testing kits, as well as the hospitals, clinics, and labs that use them to check patients.
This market has become more important, especially with the rise of respiratory illnesses in recent years. As people become more aware of these diseases and want faster results, the demand for these testing kits is growing. They are crucial in helping doctors quickly diagnose what's making someone sick, so they can start the right treatment as soon as possible.
Drivers for the Respiratory Pathogen Testing Kits Market
Growing Demand for Respiratory Pathogen Testing Kits
The Respiratory Pathogen Testing Kits market is witnessing significant growth due to several key factors. A major driver is the increasing incidence of respiratory infections worldwide, driven by factors such as pollution, climate change, and the growing population. These infections, including influenza, respiratory syncytial virus (RSV), and COVID-19, require prompt and accurate diagnosis to prevent outbreaks and manage patient care effectively. The heightened awareness and need for early detection are boosting the demand for these testing kits across various healthcare settings.
Technological Advancements in Diagnostic Tools
Advances in diagnostic technology are another crucial factor driving market growth. The development of more precise and faster diagnostic tools, such as real-time PCR (polymerase chain reaction) and next-generation sequencing (NGS), has significantly improved the ability to detect respiratory pathogens. These technologies provide quicker results with higher accuracy, making them essential in managing respiratory illnesses, especially in high-risk populations. The continuous innovation in this sector is expected to propel the market forward as healthcare providers seek more efficient and reliable testing solutions.
Increasing Healthcare Expenditure
The rise in healthcare expenditure, particularly in developed countries, is also playing a vital role in the expansion of the respiratory pathogen testing kits market. Governments and private sectors are investing heavily in healthcare infrastructure, including hospitals, clinics, and diagnostic laboratories. This investment enhances the accessibility and availability of advanced diagnostic tools, ensuring that a broader population can benefit from timely and accurate respiratory pathogen testing. The growing healthcare budgets are facilitating the adoption of these kits, particularly in regions like North America and Europe.
Expansion in Emerging Markets
Emerging markets, particularly in the Asia-Pacific region, are expected to experience the fastest growth in the respiratory pathogen testing kits market. Economic development in countries like China and India has led to increased healthcare spending and improved healthcare infrastructure. As these countries continue to develop, the demand for advanced diagnostic tools, including respiratory pathogen testing kits, is expected to rise. The increasing focus on healthcare in these regions presents a significant opportunity for market expansion.
Focus on Preventative Healthcare
There is a growing emphasis on preventative healthcare, which is further driving the demand for respiratory pathogen testing kits. With the global healthcare landscape shifting towards early detection and prevention of diseases, these kits have become essential tools in preventing the spread of respiratory infections. The focus on reducing healthcare costs by preventing severe outbreaks is encouraging the adoption of these testing kits across various healthcare settings, from hospitals to specialized clinics.
Competitive Landscape and Innovation
The competitive landscape of the respiratory pathogen testing kits market is marked by the presence of key players like Thermo Fisher Scientific, Abbott Laboratories, and Danaher Corporation. These companies are heavily investing in research and development to introduce innovative products that meet the growing demand for more accurate and faster diagnostic solutions. Strategic activities such as mergers, acquisitions, and partnerships are also common as companies aim to expand their market presence and enhance their product offerings. This competitive environment is expected to drive further innovation and growth in the market.
Restraints for the Respiratory Pathogen Testing Kits Market
Cost Constraints
One of the significant restraints on the respiratory pathogen testing kits market is the high cost associated with these diagnostic tools. Advanced technologies like RT-PCR and ELISA are commonly used in these kits, but they require expensive equipment, reagents, and skilled personnel to operate. These costs are often passed down to the end-users, such as hospitals and diagnostic centers, making the testing process costly. In regions with limited healthcare budgets, this can lead to reduced adoption rates, especially in low-income countries. Consequently, the high cost remains a major barrier to market growth.
Limited Access in Developing Regions
Another significant challenge for the market is the limited access to respiratory pathogen testing kits in developing regions. Infrastructure constraints, lack of trained healthcare professionals, and limited availability of diagnostic facilities can hinder the widespread adoption of these kits. In many developing countries, healthcare systems are underfunded, and the focus is often on more immediate healthcare needs. As a result, the market's growth potential is significantly restricted in these areas, which make up a large portion of the global population.
Regulatory Hurdles
The respiratory pathogen testing kits market also faces stringent regulatory requirements that can delay product launches and increase costs. Regulatory bodies like the FDA in the United States or the EMA in Europe have rigorous testing and approval processes to ensure the safety and effectiveness of these kits. While these regulations are crucial for maintaining high standards, they can be a double-edged sword. The lengthy approval processes can slow down the time-to-market for new products, giving rise to increased development costs and reducing the profitability for manufacturers.
Competition and Market Saturation
The market is also experiencing growing competition and potential saturation, especially in more developed regions. Numerous players, ranging from established companies like Abbott Laboratories and Thermo Fisher Scientific to smaller biotech firms, are vying for market share. This intense competition can lead to price wars, which might erode profit margins and make it difficult for smaller companies to sustain operations. Additionally, as the market becomes more saturated, the room for growth diminishes, posing a challenge for new entrants and even established players.
Supply Chain Disruptions
Finally, supply chain disruptions present another significant restraint. The production of respiratory pathogen testing kits relies on a complex global supply chain that includes raw materials, specialized components, and skilled labor. Any disruption in this chain, such as those experienced during the COVID-19 pandemic, can lead to shortages and delays. These disruptions can severely impact the market’s ability to meet demand, particularly in times of crisis, and highlight the vulnerabilities in the supply chain that need to be addressed.
Opportunity in the Respiratory Pathogen Testing Kits Market
Rising Demand for Diagnostic Accuracy
One of the key opportunities in this market is the growing emphasis on diagnostic accuracy. Healthcare providers are increasingly adopting advanced testing kits that offer high sensitivity and specificity. This trend is driven by the need to quickly and accurately identify pathogens to guide appropriate treatment, especially in high-stakes situations like pandemics. Companies that can develop and market testing kits with superior accuracy and rapid turnaround times are likely to gain a competitive edge.
Technological Advancements in Testing Kits
Technological advancements present another significant opportunity. The development of new testing technologies, such as RT-PCR, ELISA, and DFA kits, has revolutionized the way respiratory pathogens are detected. These technologies not only improve the accuracy of diagnoses but also reduce the time required to obtain results. As a result, there is a growing market for these advanced testing kits, particularly in regions with well-established healthcare infrastructures.
Expanding Healthcare Infrastructure in Emerging Markets
The expansion of healthcare infrastructure in emerging markets also provides a fertile ground for market growth. Countries in the Asia-Pacific region, such as China and India, are investing heavily in healthcare, including diagnostics. The increasing awareness about the importance of early detection and the availability of improved healthcare facilities are driving the demand for respiratory pathogen testing kits. Companies that can tap into these emerging markets stand to benefit significantly.
Increasing Government Initiatives and Funding
Government initiatives and funding aimed at improving public health and pandemic preparedness are creating additional opportunities. Many governments are allocating resources to enhance their diagnostic capabilities, particularly in the face of ongoing and future pandemics. This has led to increased procurement of respiratory pathogen testing kits by public health agencies and hospitals, boosting the overall market demand.
Rising Consumer Awareness and Preventive Health Measures
Lastly, the rise in consumer awareness about respiratory infections and the importance of early detection is contributing to market growth. As more individuals seek out testing for respiratory pathogens, either through healthcare providers or at-home testing kits, the market is expected to expand further. Companies that offer user-friendly and accessible testing solutions are likely to capture a larger share of this growing consumer market.
Trends for the Respiratory Pathogen Testing Kits Market
Growing Demand for Rapid and Accurate Testing
The respiratory pathogen testing kits market is experiencing significant growth, driven primarily by the increasing need for rapid and accurate diagnosis of respiratory infections. With the rise in respiratory diseases like COVID-19, influenza, and respiratory syncytial virus (RSV), there is a strong demand for diagnostic tools that can quickly identify these pathogens. Rapid molecular tests, including Nucleic Acid Amplification Tests (NAATs), have become essential in healthcare settings due to their high accuracy and ability to deliver results promptly. This trend is expected to continue as healthcare providers prioritize quick diagnosis to manage and treat respiratory infections effectively.
Technological Advancements Enhancing Testing Capabilities
Advancements in technology are playing a crucial role in expanding the capabilities of respiratory pathogen testing kits. Innovations such as the development of multiplex assays, which allow simultaneous detection of multiple pathogens, are streamlining the diagnostic process. These technologies are particularly beneficial in managing outbreaks of respiratory diseases by enabling faster and more comprehensive testing. Moreover, the integration of artificial intelligence and machine learning in diagnostic tools is expected to further improve the accuracy and efficiency of these tests, making them indispensable in both clinical and laboratory settings.
Increased Awareness and Accessibility
The growing awareness about the importance of early diagnosis in managing respiratory diseases is contributing to the increased adoption of testing kits. Public health campaigns and government initiatives aimed at controlling the spread of respiratory infections have led to greater utilization of these diagnostic tools. Additionally, the expansion of healthcare infrastructure, particularly in emerging markets, has made these testing kits more accessible to a broader population. This increased accessibility is likely to boost market growth, as more individuals undergo testing for respiratory pathogens.
Strategic Investments and Market Expansion
The respiratory pathogen testing kits market is witnessing strategic investments from key players aimed at expanding their product offerings and market presence. Companies are investing in research and development to introduce innovative testing solutions and are also engaging in mergers and acquisitions to strengthen their market position. This competitive landscape is fostering innovation and ensuring that a wide range of high-quality testing kits are available in the market, further driving growth.
Regional Market Dynamics
Regionally, North America is expected to dominate the respiratory pathogen testing kits market, driven by a high prevalence of respiratory diseases and a well-established healthcare infrastructure. The Asia-Pacific region is also emerging as a significant market, propelled by factors such as increasing pollution levels and the high incidence of respiratory infections. Countries like China and India are witnessing robust demand for these testing kits, supported by government initiatives and rising healthcare expenditures.
Segments Covered in the Report
By Product
o RT PCR Kits
o DFA Kits
o ELISA Kits
o Others
By Technology
o NAAT (Nucleic Acid Amplification Test)
o Immunoassays
o Others Technology
By Infection/Symptom
o Matrix
o Enterovirus Infection
o Human Coronavirus Infection
o Influenza Virus Infection
o Respiratory Syncytial Virus Infection
o Rhinovirus Infection
o Pneumonia
By End-User
o Hospitals
o Diagnostic Centers
o Specialty Clinics
o Other End-User
Segment Analysis
By Product Analysis
The Respiratory Pathogen Testing Kits Market is divided into product categories, including RT-PCR Kits, DFA Kits, and ELISA Kits. Among these, the RT-PCR Kits segment stood out as the market leader in 2023. This dominance is largely due to the exceptional accuracy and reliability of RT-PCR (Reverse Transcription Polymerase Chain Reaction) technology. RT-PCR Kits are known for their ability to detect viral RNA with unmatched sensitivity and specificity, which is crucial for identifying respiratory pathogens. These kits can detect even minimal amounts of viral genetic material, making them highly effective in diagnosing infectious diseases with great precision.
RT-PCR technology is often regarded as the "gold standard" in molecular diagnostics because it provides accurate and timely results. In the context of respiratory infections, where early detection is key to effective treatment and prevention of disease spread, RT-PCR Kits are indispensable. Their widespread adoption in clinical and laboratory settings underscores their importance in the fight against respiratory illnesses, especially in the wake of global health challenges like the COVID-19 pandemic.
Their versatility and adaptability also drive the preference for RT-PCR Kits over other testing methods. They can be used to test a wide range of respiratory pathogens, from common viruses like influenza to more complex ones like the SARS-CoV-2 virus. This versatility, combined with their precision, has solidified RT-PCR Kits' position as the leading product segment in the respiratory pathogen testing market, and this trend is likely to continue as healthcare systems prioritize accurate and reliable diagnostics.
By Technology Analysis
The Respiratory Pathogen Testing Kits Market is divided into different segments based on the technology used. The main technologies are NAAT (Nucleic Acid Amplification Test), Immunoassays, and Others. Among these, NAATs generated the highest revenue in 2023. NAATs are highly valued because they offer exceptional sensitivity and specificity when detecting the genetic material of respiratory pathogens like viruses and bacteria. This means that NAATs can accurately identify even small amounts of pathogen RNA or DNA, making them a reliable choice for diagnosing respiratory infections.
The accuracy of NAATs makes them especially useful in cases where traditional testing methods might not be as effective. For example, conventional tests might miss low levels of pathogens or produce false-negative results. NAATs overcome these limitations by amplifying the genetic material of the pathogen, which helps in detecting even minimal traces. This high level of precision is why NAATs are preferred in many diagnostic situations, ensuring that patients receive accurate results and appropriate treatment for respiratory infections.
By Infection/Symptom
The Respiratory Pathogen Testing Kits Market is segmented based on the type of infection or symptom being tested. This includes categories such as Matrix, Enterovirus Infection, Human Coronavirus Infection, Influenza Virus Infection, Respiratory Syncytial Virus Infection, Rhinovirus Infection, and Pneumonia. Among these, Enterovirus Infection was the top revenue generator in 2023. Enteroviruses are a major cause of respiratory infections, especially in children and infants. They can cause a range of illnesses from the common cold and bronchitis to more severe conditions like pneumonia and acute respiratory distress syndrome (ARDS).
Enterovirus infections are particularly prevalent in younger populations, which significantly drives the need for accurate and timely diagnostic tests. Since these infections can vary in severity, effective and early detection is crucial for proper treatment. This high demand for precise diagnostic testing has led to Enterovirus Infection becoming the leading category in revenue for respiratory pathogen testing kits. Accurate testing helps ensure that patients receive the correct diagnosis and appropriate care, making it an essential tool in managing respiratory illnesses.
By End-User Analysis
The Respiratory Pathogen Testing Kits Market is divided into different segments based on the end-users of the testing kits. These segments include Hospitals, Diagnostic Centers, and Specialty Clinics. In 2023, Hospitals were the leading revenue generators. Hospitals play a critical role in diagnosing and treating a wide range of respiratory infections caused by viruses, bacteria, and fungi. They serve as primary healthcare centers where patients with varying degrees of respiratory symptoms—from mild to severe—come for treatment.
Hospitals are on the front lines of healthcare, dealing with numerous patients who need accurate and timely diagnosis of respiratory infections. This accuracy is crucial not only for effective treatment but also for managing infection control and preventing the spread of diseases within the hospital environment. By ensuring precise diagnosis and implementing appropriate patient management and infection control measures, hospitals can reduce the risk of further transmission and complications. This high demand for effective respiratory pathogen testing in hospitals is a key reason why this sector generated the most revenue in 2023.
Regional Analysis
North American Respiratory Pathogen Testing Kits will lead this market. North America has a robust healthcare infrastructure and serves as a hub for medical technology innovation. The field is constantly investing in research and improvement, particularly in the development of cutting-edge breathing pathogens and the testing of kits with increased accuracy, sensitivity, and speed. Technological advancements in molecular diagnostics, such as real-time PCR (polymerase chain reaction) and next-generation sequencing, have significantly increased the capabilities of respiratory pathogens testing, resulting in market dominance.
The European Respiratory Pathogen Testing Kits market has the second-largest market share. Europe has a well-developed healthcare infrastructure, including superior scientific facilities, research institutes, and diagnostic laboratories. This infrastructure allows for widespread access to respiratory pathogens while testing services around the area. European foreign locations prioritize healthcare spending, particularly in the use of modern diagnostic technology and testing procedures. The availability of modern healthcare infrastructure promotes the expansion of the breathing pathogen, as evidenced by the market in Europe. Furthermore, the German Respiratory Pathogen Testing Kits market had the highest market share, while the UK Respiratory Pathogen Testing Kits market was the fastest expanding in the European area.
Competitive Analysis
Leading market companies are extensively spending on R&D to extend their product lines, allowing the Respiratory Pathogen Testing Kits market to grow even more. Market participants are also engaging in several strategic initiatives to grow their worldwide presence, with significant market developments including new product launches, contractual agreements, mergers and acquisitions, increased investments, and collaboration with other organizations. To grow and compete in a more competitive and expanding market, the Respiratory Pathogen Testing Kits industry must provide affordable products.
Recent Developments
In November 2021: Philips enhanced their ultrasound solution for Radiology with more sophisticated imaging tools and features, hence boosting workflow effectiveness and diagnostic certainty.
In February 2021: Mesa Biotech was acquired by Thermo Fisher to help expand its Life Sciences Solutions business line. This transaction is expected to increase revenue for this segment by roughly $200 million in 2021.
Key Market Players in the Respiratory Pathogen Testing Kits Market
o Danaher Corporation
o Becton, Dickinson and Company
o Altona Diagnostics
o MyLab
o Abbott Laboratories
o Hoffmann-La Roche Ltd.
o Other Key Players
|
Report Features |
Description |
|
Market Size 2023 |
USD 2.8 Billion |
|
Market Size 2033 |
USD 4.9 Billion |
|
Compound Annual Growth Rate (CAGR) |
5.7% (2023-2033) |
|
Base Year |
2023 |
|
Market Forecast Period |
2024-2033 |
|
Historical Data |
2019-2023 |
|
Market Forecast Units |
Value (USD Billion) |
|
Report Coverage |
Revenue Forecast, Market Competitive Landscape, Growth Factors, and Trends |
|
Segments Covered |
By Product, Technology, Infection/Symptom, End-User, and Region |
|
Geographies Covered |
North America, Europe, Asia Pacific, and the Rest of the World |
|
Countries Covered |
The U.S., Canada, Germany, France, U.K, Italy, Spain, China, Japan, India, Australia, South Korea, and Brazil |
|
Key Companies Profiled |
Danaher Corporation, Becton, Dickinson and Company, bioMérieux Inc., Altona Diagnostics, MyLab, Abbott Laboratories, Thermo Fisher Scientific, Hoffmann-La Roche Ltd., Other Key Players |
|
Key Market Opportunities |
Technological Advancements in Testing Kits |
|
Key Market Dynamics |
Growing Demand for Respiratory Pathogen Testing Kits |
📘 Frequently Asked Questions
1. How much is the Respiratory Pathogen Testing Kits Market in 2023?
Answer: The Respiratory Pathogen Testing Kits Market size was valued at USD 2.8 Billion in 2023.
2. What would be the forecast period in the Respiratory Pathogen Testing Kits Market?
Answer: The forecast period in the Respiratory Pathogen Testing Kits Market report is 2023-2033.
3. Who are the key players in the Respiratory Pathogen Testing Kits Market?
Answer: Danaher Corporation, Becton, Dickinson and Company, bioMérieux Inc., Altona Diagnostics, MyLab, Abbott Laboratories, Thermo Fisher Scientific, Hoffmann-La Roche Ltd., Other Key Players
4. What is the growth rate of the Respiratory Pathogen Testing Kits Market?
Answer: Respiratory Pathogen Testing Kits Market is growing at a CAGR of 5.7% during the forecast period, from 2023 to 2033.

🔐 Secure Payment Guaranteed
Safe checkout with trusted global payment methods.
🌟 Why Choose Infinity Market Research?
- Accurate & Verified Data:Our insights are trusted by global brands and Fortune 500 companies.
- Complete Transparency:No hidden fees, locked content, or misleading claims — ever.
- 24/7 Analyst Support:Our expert team is always available to help you make smarter decisions.
- Instant Savings:Enjoy a flat $1000 OFF on every report.
- Fast & Reliable Delivery:Get your report delivered within 5 working days, guaranteed.
- Tailored Insights:Customized research that fits your industry and specific goals.



